Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Sulphur dioxide is to be removed from air by contacting the gas with water in a column packed with 34-in ceramic Berl saddles. The entering
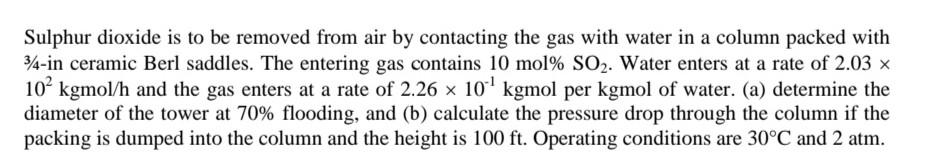
Sulphur dioxide is to be removed from air by contacting the gas with water in a column packed with 34-in ceramic Berl saddles. The entering gas contains 10 mol% SO2. Water enters at a rate of 2.03 x 10 kgmol/h and the gas enters at a rate of 2.26 x 10 kgmol per kgmol of water. (a) determine the diameter of the tower at 70% flooding, and (b) calculate the pressure drop through the column if the packing is dumped into the column and the height is 100 ft. Operating conditions are 30C and 2 atm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


