Question
Using the entropy change equation of the lattice model, calculate the change in entropy for the following mixtures. (a) 300 g of toluene and
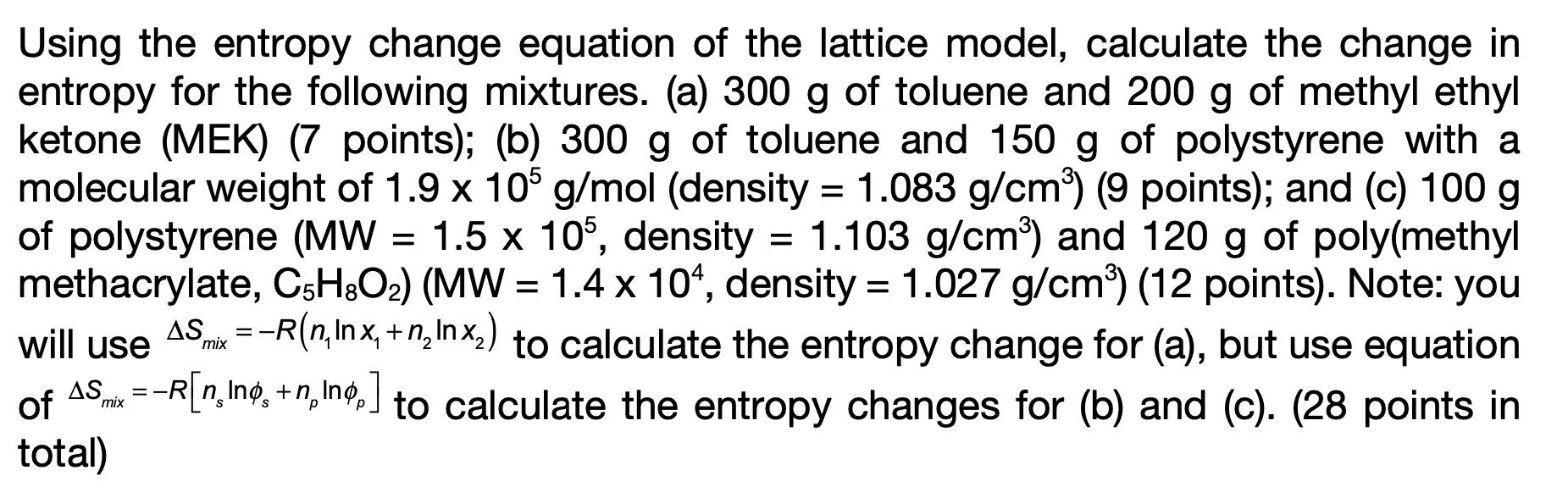
Using the entropy change equation of the lattice model, calculate the change in entropy for the following mixtures. (a) 300 g of toluene and 200 g of methyl ethyl ketone (MEK) (7 points); (b) 300 g of toluene and 150 g of polystyrene with a molecular weight of 1.9 x 105 g/mol (density = 1.083 g/cm) (9 points); and (c) 100 g of polystyrene (MW = 1.5 x 105, density = 1.103 g/cm) and 120 g of poly(methyl methacrylate, C5H8O) (MW = 1.4 x 104, density = 1.027 g/cm) (12 points). Note: you will use AS mix=-R(n, Inx,+nInx) to calculate the entropy change for (a), but use equation =-R[nIng+ning,] to calculate the entropy changes for (b) and (c). (28 points in AS of total) mix
Step by Step Solution
There are 3 Steps involved in it
Step: 1
300g of toluene and 200g of methyl ethyl ketone RM...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Operations management processes and supply chain
Authors: Lee J Krajewski, Larry P Ritzman, Manoj K Malhotra
9th edition
9788131728840, 136065767, 8131728846, 978-0136065760
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



