Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Suppose the pressure is 3.0 atm, and calculate the heat of reaction where hydrogen is combusted in the engine at 100C,3.0atm. 2H2 (g) + O2
Suppose the pressure is 3.0 atm, and calculate the heat of reaction where hydrogen is combusted in the engine at 100C,3.0atm.
2H2 (g) + O2 (g) 2H2O(l) 
The initial condition is in standard state.
The final condition is 90C,3atm.


The question need to be edit not 100C, it need to be 90C.
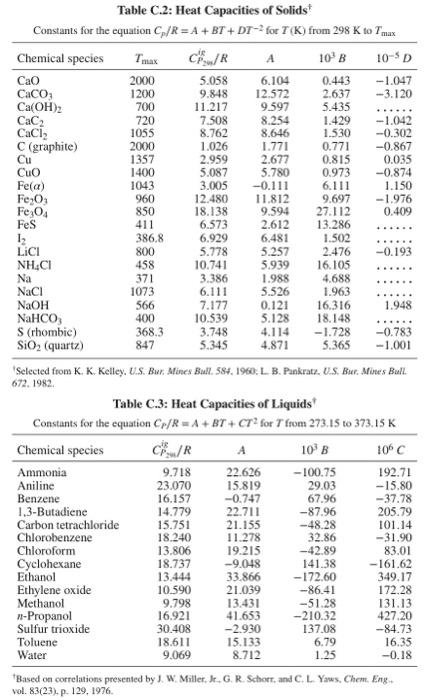
Table C.1: Heat Capacities of Gases in the Ideal-Gas State? Constants in equation CPig/R=A+BT+CT2+DT2 for T(K) from 298K to Tmax Table C.2: Heat Capacities of Solids Constants for the equation Cp/R=A+BT+DT2 for T(K) from 298K to Tmax 'Selected from K. K. Kelley. U.S. Bur. Mines Bull. 584, 1960, L. B. Pankratz. U.S. Bur, Mines Bull. 672.1982. Table C.3: Heat Capacities of Liquids Constants for the equation CP/R=A+BT+CT2 for T from 273.15 to 373.15K 'Based on correlations presented by J. W. Miller, Jr. G. R. Sehorr, and C. L. Yaws, Chem. Eng. vol. 83(23), p. 129,1976 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


