Answered step by step
Verified Expert Solution
Question
1 Approved Answer
suppose you have 2.50l of a substance with a density of 4.00 g/ml and you want to determjme the mass of the substance. Suppose you
suppose you have 2.50l of a substance with a density of 4.00 g/ml and you want to determjme the mass of the substance.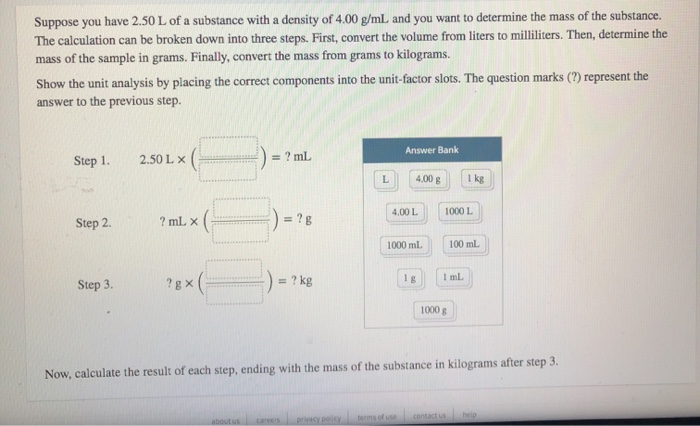

Suppose you have 2.50 L of a substance with a density of 4.00 g/mL and you want to determine the mass of the substance. The calculation can be broken down into three steps. First, convert the volume from liters to milliliters. Then, determine the mass of the sample in grams. Finally, convert the mass from grams to kilograms. Show the unit analysis by placing the correct components into the unit-factor slots. The question marks (?) represent the answer to the previous step. Step 1. Step 2. Step 3. 2.50 L X ? mL X ?gx = ? mL about us = ?g = ? kg careers L Answer Bank 4.00 g 4.00 L 1000 ml. privacy policy terms of use 1g 1000 L 1 mL 1000 g Now, calculate the result of each step, ending with the mass of the substance in kilograms after step 3. 1 kg contact us 100 mL help
Step by Step Solution
★★★★★
3.45 Rating (171 Votes )
There are 3 Steps involved in it
Step: 1
Given that Volume of substance V De...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


