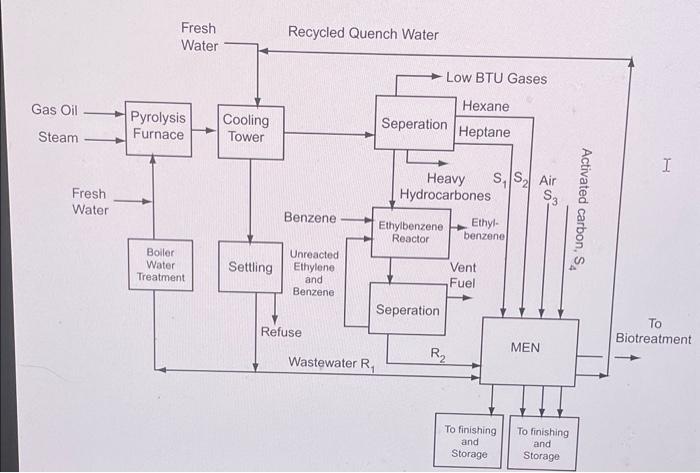
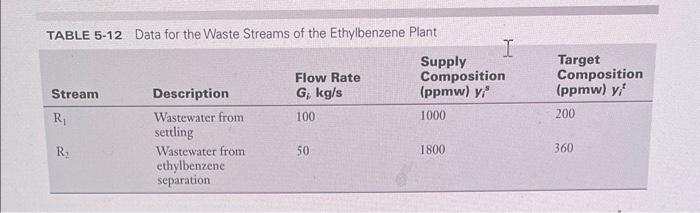
Sustainable Design through process integration subject 5. Fig. 5.34 shows the process flow sheet for an ethylene/ethylbenzene plant (Stanley and El-Halwagi, 1995). Gas oil is cracked with steam in a pyrolysis furnace to form ethylene, low BTU gases, hexane, heptane, and heavier hydrocarbons. The ethylene is then reacted with benzene to form ethylbenzene. Two wastewater streams are formed: R1, which is the quench water recycle for the cooling tower, and R2, which is the wastewater from the ethylbenzene portion of the plant. The primary pollutant present in the two wastewater streams is benzene. Benzene must be removed from stram R1 down to a concentration of 200ppm before R1 can be recycled back to the cooling tower. Benzene must also be removed from stream R2 down to a concentration of 360ppm before R2 can be sent to biotreatment. The data for streams R1 and R2 are shown in Table 5.12. There are two process MSAs available to remove benzene from the wastewater streams. These process MSAs are hexane (S1) and heptane (S2). Hexane is available at a flow rate of 0.8kg/s and supply composition of 10ppmw whereas heptane is available at a flow rate of 0.3kg/s and supply composition of 15 ppmw. The target compositions for hexane and heptane are unknown and should be determined by the engineer designing the MEN. The mass-transfer driving forces, 1 and 2, should be at least 30,000 and 20,000ppmw, respectively. The equilibrium data for benzene transfer from wastewater to hexane and heptane are: [5.34]y=0.011x1 and [5.35] y=0.008x2 where y,x1, and x2 are given in mass fractions. Two external MSAs are considered for removing benzene: air (S3) and activated carbon (S4). Air is compressed to 3atm before stripping. Following stripping, benzene is separated from air using condensation. Henry's law can be used to predict equilibrium for the stripping process. Activated carbon is continuously regenerated using steam in the ratio of 1.5kg steam: 1kg of benzene adsorbed on activated carbon. Makeup at the rate of 1 percent of recirculating activated carbon is needed to compensate for losses due to regeneration and deactivation. Over the operating range, the equilibrium relation for the transfer of benzene from wastewater onto activated carbon can be described by: [5.36] y=7.0104x4 1. Using the pinch diagram, determine the pinch location, minimum load of benzene to be removed by external MSAs, and excess capacity of process MSAs. How do you remove this excess capacity? 2. Consideringt the four candidate MSAs, what is the MOC needed to remove benzene? Itak-Fik. IHon-man Dtane