Answered step by step
Verified Expert Solution
Question
1 Approved Answer
T=800 C- a Si T Xmax a + 1 1/2 X = Xp P GS I Si Xmax T = 800 C 1/ X
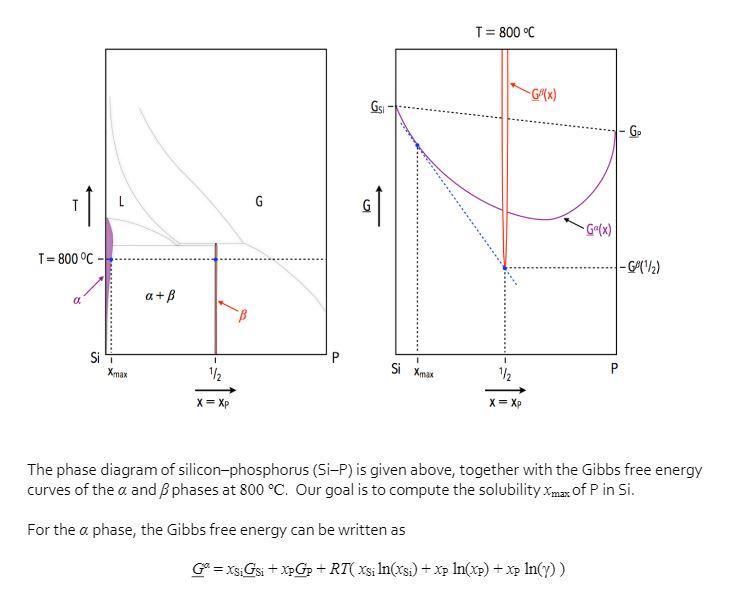
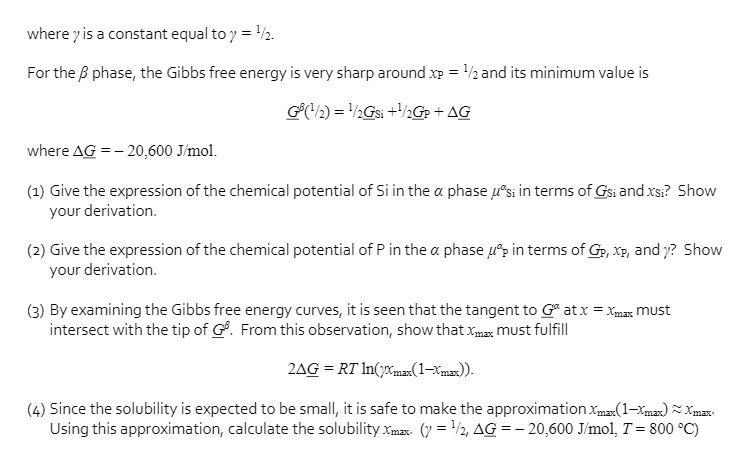
T=800 C- a Si T Xmax a + 1 1/2 X = Xp P GS I Si Xmax T = 800 C 1/ X = Xp -G(x) -Ga(x) G = XsiGsi + XpGp + RT(xsi ln(xsi) + xp ln(xp) + xp ln(y)) P Go -G (/2) The phase diagram of silicon-phosphorus (Si-P) is given above, together with the Gibbs free energy curves of the a and 3 phases at 800 C. Our goal is to compute the solubility Xmax of P in Si. For the a phase, the Gibbs free energy can be written as where y is a constant equal to y = 2. For the phase, the Gibbs free energy is very sharp around xp = /2 and its minimum value is GB(/2) = /2Gsi +/2Gp + AG where AG = -20,600 J/mol. (1) Give the expression of the chemical potential of Si in the a phase usi in terms of Gsi and xsi? Show your derivation. (2) Give the expression of the chemical potential of P in the a phase up in terms of Gp, xp, and y? Show your derivation. (3) By examining the Gibbs free energy curves, it is seen that the tangent to Gat x = xmax must intersect with the tip of G. From this observation, show that Xxmax must fulfill 2AG = RT In(max(1-Xmax)). (4) Since the solubility is expected to be small, it is safe to make the approximation Xmax(1-Xmax)~Xmax Using this approximation, calculate the solubility Xmax (y=/2, AG = -20,600 J/mol, T = 800 C)
Step by Step Solution
★★★★★
3.52 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
1 The chemical potential of Si in the a phase si can be derived from the expression of Gibbs free energy as follows G12 12GSi 12Gp AG Since G12 represents the minimum value of the Gibbs free energy fo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


