Question
T3T3. The process B -> C shown above is A. An isochoric process B. An isothermal process C. An isobaric process D. An adiabatic process
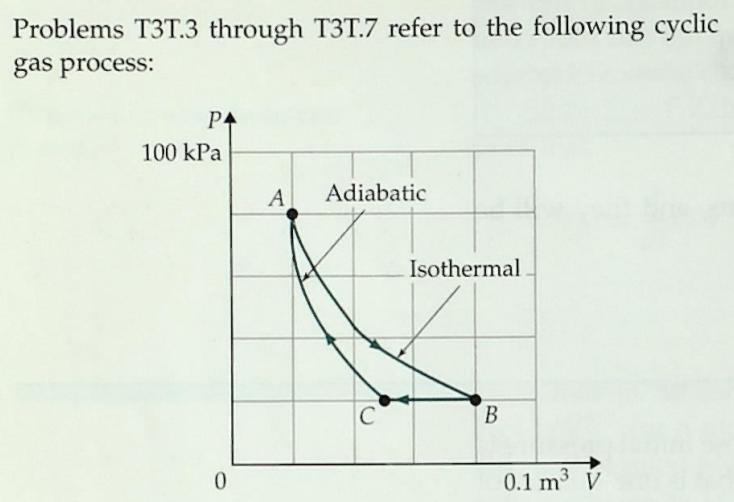
T3T3. The process B -> C shown above is
A. An isochoric process
B. An isothermal process
C. An isobaric process
D. An adiabatic process
E. An isometric process
F. None of the above
T3T4. Is the work energy flowing into or out of the gas in
process B -> C positive (A) or negative(B)?
Which of the values below is closest to the magnitude of W?
A. 0.6 J
B. 1.5 J
C. 300 J
D. 600 J
E. 1500 J
F. 3000 J
T3T5. What are the signs of Q, W, and delta U for the process
C -> A?
A. +, - , 0
B. 0, - , -
C. 0, + , +
D. + , + , 0
Problems T3T.3 through T3T.7 refer to the following cyclic gas process: P4 100 kPa A Adiabatic Isothermal C B 0.1 m V
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Interactive Approach
Authors: Subrata Bhattacharjee
1st edition
130351172, 978-0130351173
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



