Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Table 6.1 Please explain the question step by step not in short answer 1. Consider that a known quantity (0.001 mole) of acid/base/salt is added
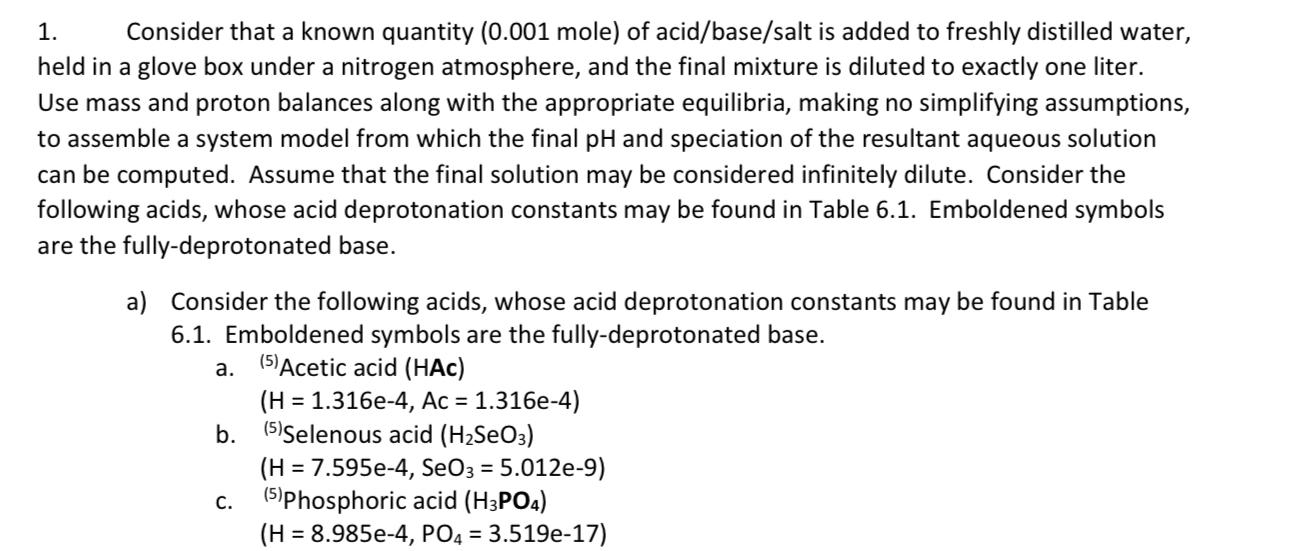
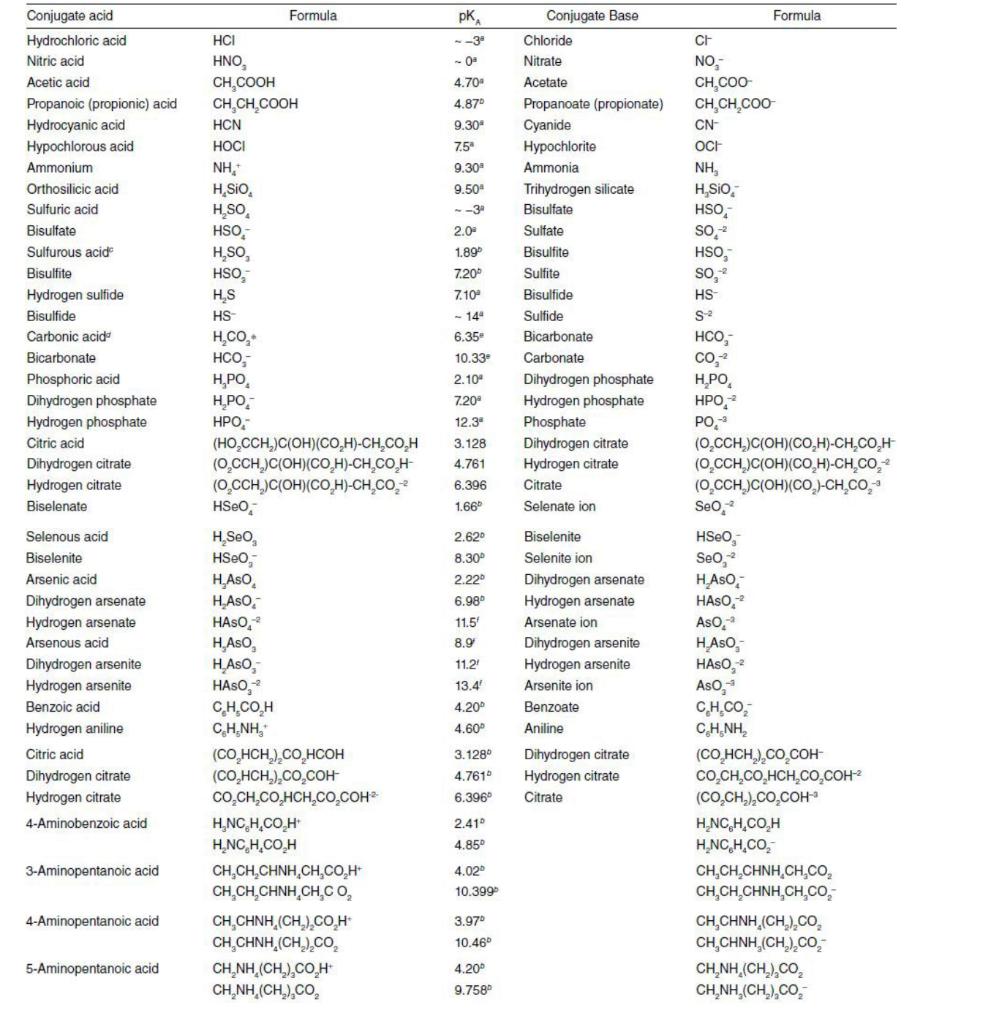
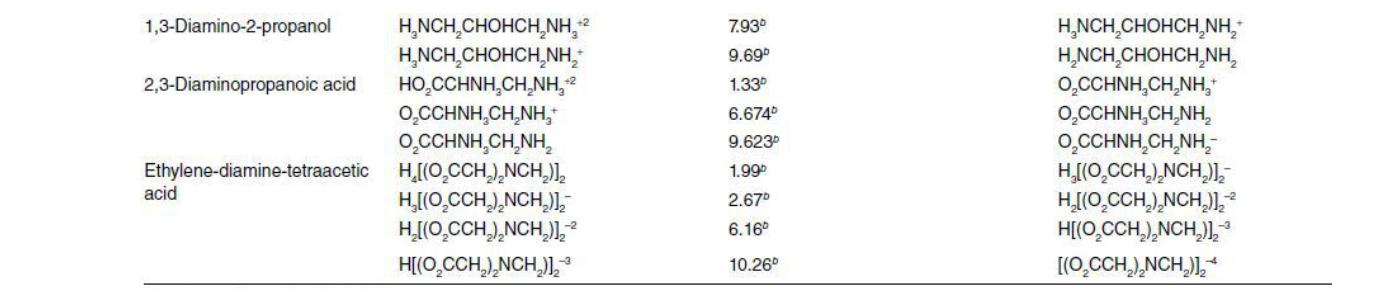 Table 6.1
Table 6.1
Please explain the question step by step not in short answer
1. Consider that a known quantity (0.001 mole) of acid/base/salt is added to freshly distilled water, held in a glove box under a nitrogen atmosphere, and the final mixture is diluted to exactly one liter. Use mass and proton balances along with the appropriate equilibria, making no simplifying assumptions, to assemble a system model from which the final pH and speciation of the resultant aqueous solution can be computed. Assume that the final solution may be considered infinitely dilute. Consider the following acids, whose acid deprotonation constants may be found in Table 6.1. Emboldened symbols are the fully-deprotonated base. a. = a) Consider the following acids, whose acid deprotonation constants may be found in Table 6.1. Emboldened symbols are the fully-deprotonated base. (5) Acetic acid (HAC) (H = 1.316e-4, Ac = 1.316e-4) b. (5)Selenous acid (H2SeO3) (H = 7.595e-4, SeO3 = 5.012e-9) (5)Phosphoric acid (H3PO4) (H = 8.985e-4, PO4 = 3.519e-17) C. Formula , Formula --0- 4.70 4.87 9.30 7.5 9.30 Conjugate acid Hydrochloric acid Nitric acid Acetic acid Propanoic (propionic) acid Hydrocyanic acid Hypochlorous acid Ammonium Orthosilicic acid Sulfuric acid Bisulfate Sulfurous acide Bisulfite Hydrogen sulfide Bisulfide Carbonic acide Bicarbonate Phosphoric acid Dihydrogen phosphate Hydrogen phosphate Citric acid Dihydrogen citrate Hydrogen citrate Biselenate HCI , CH,COOH CH,CH,COOH HCN HOCI NH, H, SIO H.SO HSO, H,SO, HSO, HS HS H.CO, HCO, H,PO H.PO HPO, (HO,CCH, C(OH)(COH)-CH_CO_H (OCCH2)C(OH)(COH)-CH COH- (O_CCH)C(OH)(COH)-CH CO.* HSEO 9.50 --3 2.0- 1.89 7.20 7.10 - 14" 6.35 10.33 2.10 7.20 12.3" 3.128 4.761 6.396 1.66 Conjugate Base Chloride Nitrate Acetate Propanoate (propionate) Cyanide Hypochlorite Ammonia Trihydrogen silicate Bisulfate Sulfate Bisulfite Sulfite Bisulfide Sulfide Bicarbonate Carbonate Dihydrogen phosphate Hydrogen phosphate Phosphate Dihydrogen citrate Hydrogen citrate Citrate Selenate ion CH NO, CH,COO CH,CH,COO CN- OCH NH, H, SIO HSO, SO. HSO, So, HS S2 HCO, CO. H.PO HPO, PO, (O_CCH2)C(OH)(COH)-CH_CO,H (OCCH)C(OH)(CO,H)-CH.CO, (OCCH.)C(OH)(CO)-CH.CO, Seo, HSeo Seo, 2.62 8.30 2.22 6.98 HASO Selenous acid Biselenite Arsenic acid Dihydrogen arsenate Hydrogen arsenate Arsenous acid Dihydrogen arsenite Hydrogen arsenite Benzoic acid Hydrogen aniline Citric acid Dihydrogen citrate Hydrogen citrate 4-Aminobenzoic acid 11.5 8.9 11.2 13.4 4.20 4.60 Biselenite Selenite ion Dihydrogen arsenate Hydrogen arsenate Arsenate ion Dihydrogen arsenite Hydrogen arsenite Arsenite ion Benzoate Aniline H, Seo HSEO, H.As. HASO HASO, H, Aso H.Aso, HASO, C.H.COH CH, NH." (CO HCH),CO, HCOH (CO,HCH.),CO,COH CO,CH,CO,HCH,CO,COH H.NCH.COH H.NC, H.CO.H CH,CH,CHNH,CH,COH CH CHCHNH CHC CH CHINH (CH2)COH CH,CHNH (CH2)2CO, CHNH (CH) COH CHNH (CH, CO, 3.128 4.7610 6.396 Dihydrogen citrate Hydrogen citrate Citrate HASO-2 Aso, HASO, HASO, Aso, CH.COM CHNH, (CO, HCH),CO,COH CO,CH,CO,HCH,CO,COH (CO,CH.),CO,COH HNC HCOH HANCH.CO, CH,CHCHNHCH CO, CHCH CHNH,CH C CH,CHNH (CH2)2CO, CH,CHNH (CH2)CO, CHNH (CH, CO, CHNH (CH2)CO, 2.410 4.85 3-Aminopentanoic acid 4.02 10.399 4-Aminopentanoic acid 3.97 10.46 5-Aminopentanoic acid 4.20 9.758 7.93 9.69 1.33 6.6740 1,3-Diamino-2-propanol H.NCH,CHOHCH,NH,2 H,NCH,CHOHCH, NH, 2,3-Diaminopropanoic acid HO CCHNH CHNH, OCCHNH CHNH, OCCHNH CHNH, Ethylene-diamine-tetraacetic HIO CCH),NCH)], H,[O_CCH),NCH)], H[(O,CCH), NCH)], H[(O CCH),NCH)], 9.623 H,NCH,CHOHCH NH; H,NCH,CHOHCH,NH O,CCHNH CHNH." 0,CCHNH,CH,NH, OCCHNH,CH_NH; H[O_CCH),NCH)] H[CO_CCH),NCH)], H[(O_CCH),NCH)] [(O CCH),NCH)], acid 1.99 2.67 6.16 10.26Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


