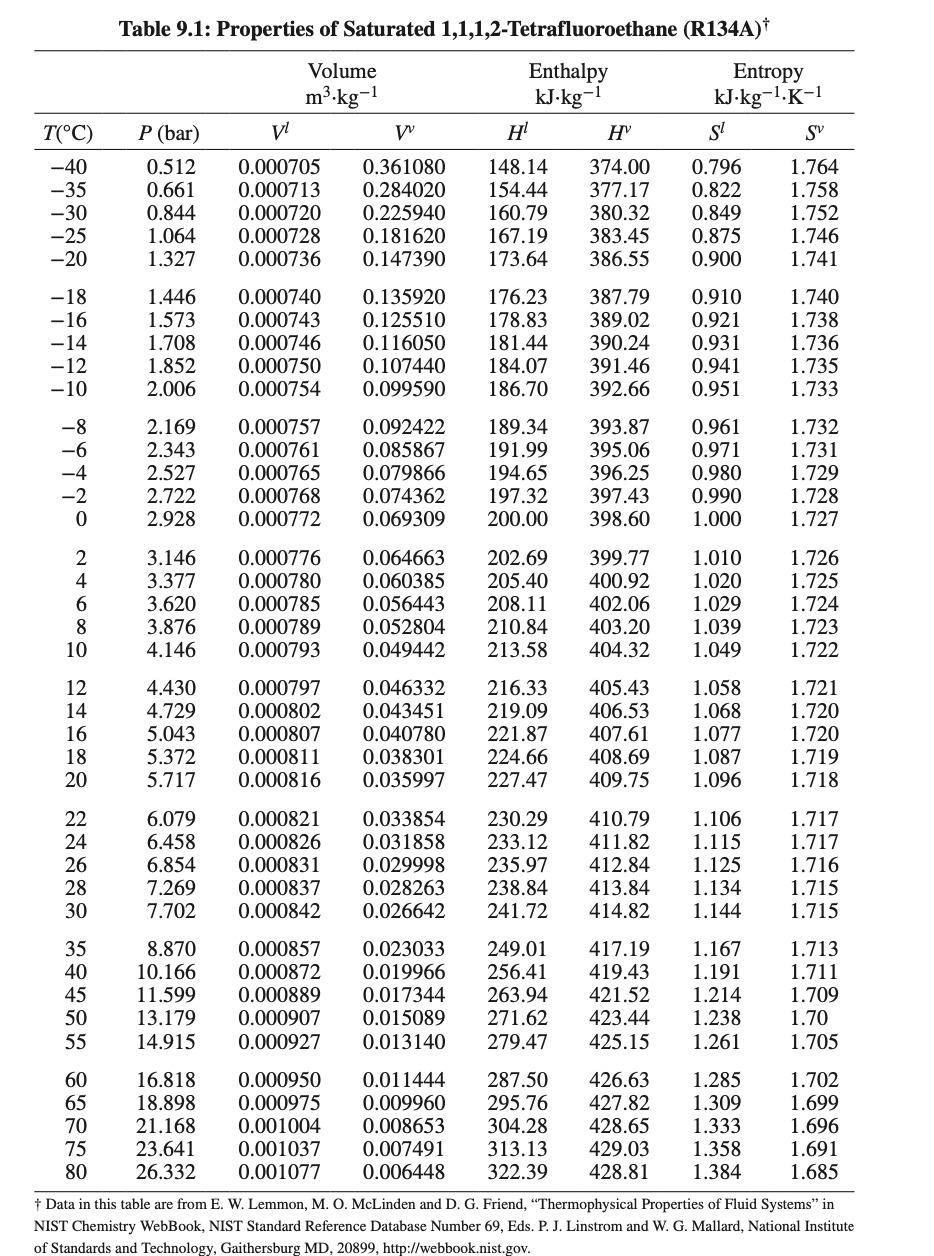
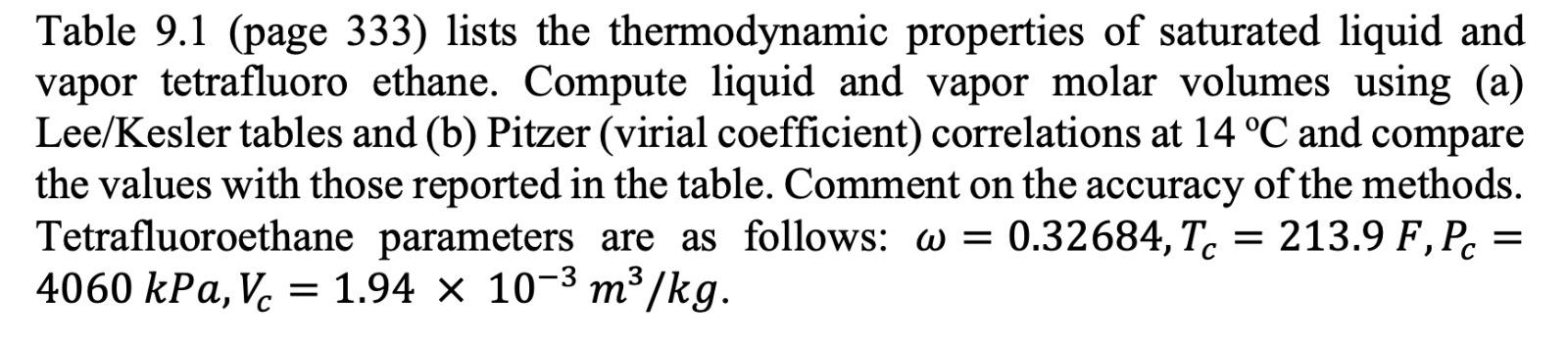
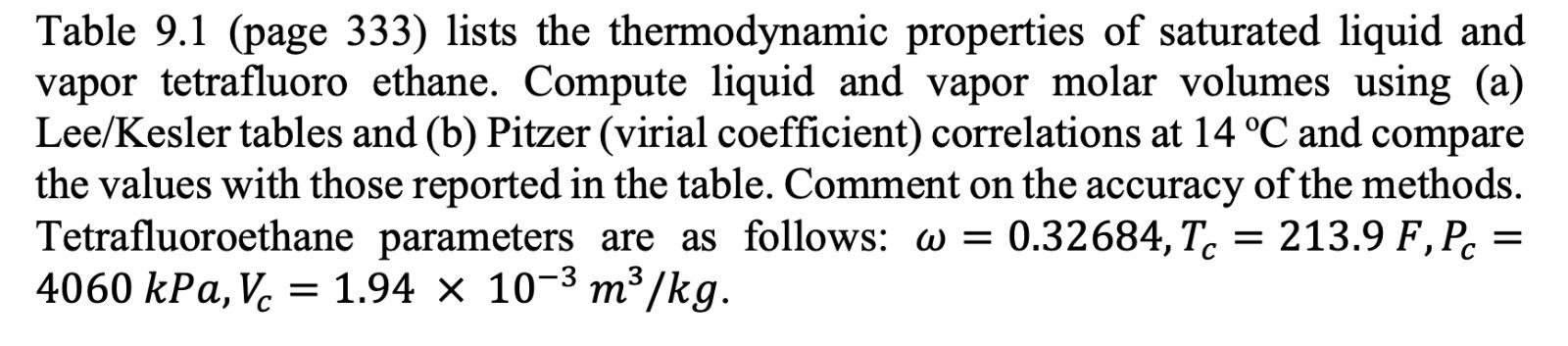
Table 9.1: Properties of Saturated 1,1,1,2-Tetrafluoroethane (R134A)* Enthalpy Volume m.kg-1 kJ kg-1 P (bar) V w H HY T(C) -40 -35 -30 -25 -20 0.512 0.661 0.844 1.064 1.327 0.000705 0.000713 0.000720 0.000728 0.000736 0.361080 0.284020 0.225940 0.181620 0.147390 148.14 154.44 160.79 167.19 173.64 374.00 377.17 380.32 383.45 386.55 Entropy kJ kg-1.K-1 S! SY 0.796 1.764 0.822 1.758 0.849 1.752 0.875 1.746 0.900 1.741 -18 -16 -14 -12 -10 1.446 1.573 1.708 1.852 2.006 0.000740 0.000743 0.000746 0.000750 0.000754 0.135920 0.125510 0.116050 0.107440 0.099590 176.23 178.83 181.44 184.07 186.70 387.79 389.02 390.24 391.46 392.66 0.910 0.921 0.931 0.941 0.951 1.740 1.738 1.736 1.735 1.733 -8 -6 -4 -2 0 2.169 2.343 2.527 2.722 2.928 0.000757 0.000761 0.000765 0.000768 0.000772 0.092422 0.085867 0.079866 0.074362 0.069309 189.34 191.99 194.65 197.32 200.00 393.87 395.06 396.25 397.43 398.60 0.961 0.971 0.980 0.990 1.000 1.732 1.731 1.729 1.728 1.727 2 4 6 8 10 3.146 3.377 3.620 3.876 4.146 0.000776 0.000780 0.000785 0.000789 0.000793 0.064663 0.060385 0.056443 0.052804 0.049442 202.69 205.40 208.11 210.84 213.58 399.77 400.92 402.06 403.20 404.32 1.010 1.020 1.029 1.039 1.049 1.726 1.725 1.724 1.723 1.722 12 14 16 18 20 4.430 4.729 5.043 5.372 5.717 0.000797 0.000802 0.000807 0.000811 0.000816 0.046332 0.043451 0.040780 0.038301 0.035997 216.33 219.09 221.87 224.66 227.47 405.43 406.53 407.61 408.69 409.75 1.058 1.068 1.077 1.087 1.096 1.721 1.720 1.720 1.719 1.718 22 24 26 28 30 6.079 6.458 6.854 7.269 7.702 0.000821 0.000826 0.000831 0.000837 0.000842 0.033854 0.031858 0.029998 0.028263 0.026642 230.29 233.12 235.97 238.84 241.72 410.79 411.82 412.84 413.84 414.82 1.106 1.115 1.125 1.134 1.144 1.717 1.717 1.716 1.715 1.715 35 40 45 50 55 8.870 10.166 11.599 13.179 14.915 0.000857 0.000872 0.000889 0.000907 0.000927 0.023033 0.019966 0.017344 0.015089 0.013140 249.01 256.41 263.94 271.62 279.47 417.19 419.43 421.52 423.44 425.15 1.167 1.191 1.214 1.238 1.261 1.713 1.711 1.709 1.70 1.705 60 16.818 0.000950 0.011444 287.50 426.63 1.285 1.702 65 18.898 0.000975 0.009960 295.76 427.82 1.309 1.699 70 21.168 0.001004 0.008653 304.28 428.65 1.333 1.696 75 23.641 0.001037 0.007491 313.13 429.03 1.358 1.691 80 26.332 0.001077 0.006448 322.39 428.81 1.384 1.685 + Data in this table are from E. W. Lemmon, M. O. McLinden and D. G. Friend, Thermophysical Properties of Fluid Systems in NIST Chemistry WebBook, NIST Standard Reference Database Number 69, Eds. P. J. Linstrom and W. G. Mallard, National Institute of Standards and Technology, Gaithersburg MD, 20899, http://webbook.nist.gov. Table 9.1 (page 333) lists the thermodynamic properties of saturated liquid and vapor tetrafluoro ethane. Compute liquid and vapor molar volumes using (a) Lee/Kesler tables and (b) Pitzer (virial coefficient) correlations at 14 C and compare the values with those reported in the table. Comment on the accuracy of the methods. Tetrafluoroethane parameters are as follows: w = 0.32684, Tc = 213.9 F, Pc = 4060 kPa, V. = 1.94 x 10-3 m3/kg. Vc = = Table 9.1 (page 333) lists the thermodynamic properties of saturated liquid and vapor tetrafluoro ethane. Compute liquid and vapor molar volumes using (a) Lee/Kesler tables and (b) Pitzer (virial coefficient) correlations at 14 C and compare the values with those reported in the table. Comment on the accuracy of the methods. Tetrafluoroethane parameters are as follows: w = 0.32684, Tc = 213.9 F, Pc = 4060 kPa, V. = 1.94 x 10-3 m3/kg. Vc = = Table 9.1: Properties of Saturated 1,1,1,2-Tetrafluoroethane (R134A)* Enthalpy Volume m.kg-1 kJ kg-1 P (bar) V w H HY T(C) -40 -35 -30 -25 -20 0.512 0.661 0.844 1.064 1.327 0.000705 0.000713 0.000720 0.000728 0.000736 0.361080 0.284020 0.225940 0.181620 0.147390 148.14 154.44 160.79 167.19 173.64 374.00 377.17 380.32 383.45 386.55 Entropy kJ kg-1.K-1 S! SY 0.796 1.764 0.822 1.758 0.849 1.752 0.875 1.746 0.900 1.741 -18 -16 -14 -12 -10 1.446 1.573 1.708 1.852 2.006 0.000740 0.000743 0.000746 0.000750 0.000754 0.135920 0.125510 0.116050 0.107440 0.099590 176.23 178.83 181.44 184.07 186.70 387.79 389.02 390.24 391.46 392.66 0.910 0.921 0.931 0.941 0.951 1.740 1.738 1.736 1.735 1.733 -8 -6 -4 -2 0 2.169 2.343 2.527 2.722 2.928 0.000757 0.000761 0.000765 0.000768 0.000772 0.092422 0.085867 0.079866 0.074362 0.069309 189.34 191.99 194.65 197.32 200.00 393.87 395.06 396.25 397.43 398.60 0.961 0.971 0.980 0.990 1.000 1.732 1.731 1.729 1.728 1.727 2 4 6 8 10 3.146 3.377 3.620 3.876 4.146 0.000776 0.000780 0.000785 0.000789 0.000793 0.064663 0.060385 0.056443 0.052804 0.049442 202.69 205.40 208.11 210.84 213.58 399.77 400.92 402.06 403.20 404.32 1.010 1.020 1.029 1.039 1.049 1.726 1.725 1.724 1.723 1.722 12 14 16 18 20 4.430 4.729 5.043 5.372 5.717 0.000797 0.000802 0.000807 0.000811 0.000816 0.046332 0.043451 0.040780 0.038301 0.035997 216.33 219.09 221.87 224.66 227.47 405.43 406.53 407.61 408.69 409.75 1.058 1.068 1.077 1.087 1.096 1.721 1.720 1.720 1.719 1.718 22 24 26 28 30 6.079 6.458 6.854 7.269 7.702 0.000821 0.000826 0.000831 0.000837 0.000842 0.033854 0.031858 0.029998 0.028263 0.026642 230.29 233.12 235.97 238.84 241.72 410.79 411.82 412.84 413.84 414.82 1.106 1.115 1.125 1.134 1.144 1.717 1.717 1.716 1.715 1.715 35 40 45 50 55 8.870 10.166 11.599 13.179 14.915 0.000857 0.000872 0.000889 0.000907 0.000927 0.023033 0.019966 0.017344 0.015089 0.013140 249.01 256.41 263.94 271.62 279.47 417.19 419.43 421.52 423.44 425.15 1.167 1.191 1.214 1.238 1.261 1.713 1.711 1.709 1.70 1.705 60 16.818 0.000950 0.011444 287.50 426.63 1.285 1.702 65 18.898 0.000975 0.009960 295.76 427.82 1.309 1.699 70 21.168 0.001004 0.008653 304.28 428.65 1.333 1.696 75 23.641 0.001037 0.007491 313.13 429.03 1.358 1.691 80 26.332 0.001077 0.006448 322.39 428.81 1.384 1.685 + Data in this table are from E. W. Lemmon, M. O. McLinden and D. G. Friend, Thermophysical Properties of Fluid Systems in NIST Chemistry WebBook, NIST Standard Reference Database Number 69, Eds. P. J. Linstrom and W. G. Mallard, National Institute of Standards and Technology, Gaithersburg MD, 20899, http://webbook.nist.gov. Table 9.1 (page 333) lists the thermodynamic properties of saturated liquid and vapor tetrafluoro ethane. Compute liquid and vapor molar volumes using (a) Lee/Kesler tables and (b) Pitzer (virial coefficient) correlations at 14 C and compare the values with those reported in the table. Comment on the accuracy of the methods. Tetrafluoroethane parameters are as follows: w = 0.32684, Tc = 213.9 F, Pc = 4060 kPa, V. = 1.94 x 10-3 m3/kg. Vc = = Table 9.1 (page 333) lists the thermodynamic properties of saturated liquid and vapor tetrafluoro ethane. Compute liquid and vapor molar volumes using (a) Lee/Kesler tables and (b) Pitzer (virial coefficient) correlations at 14 C and compare the values with those reported in the table. Comment on the accuracy of the methods. Tetrafluoroethane parameters are as follows: w = 0.32684, Tc = 213.9 F, Pc = 4060 kPa, V. = 1.94 x 10-3 m3/kg. Vc = =









