Answered step by step
Verified Expert Solution
Question
1 Approved Answer
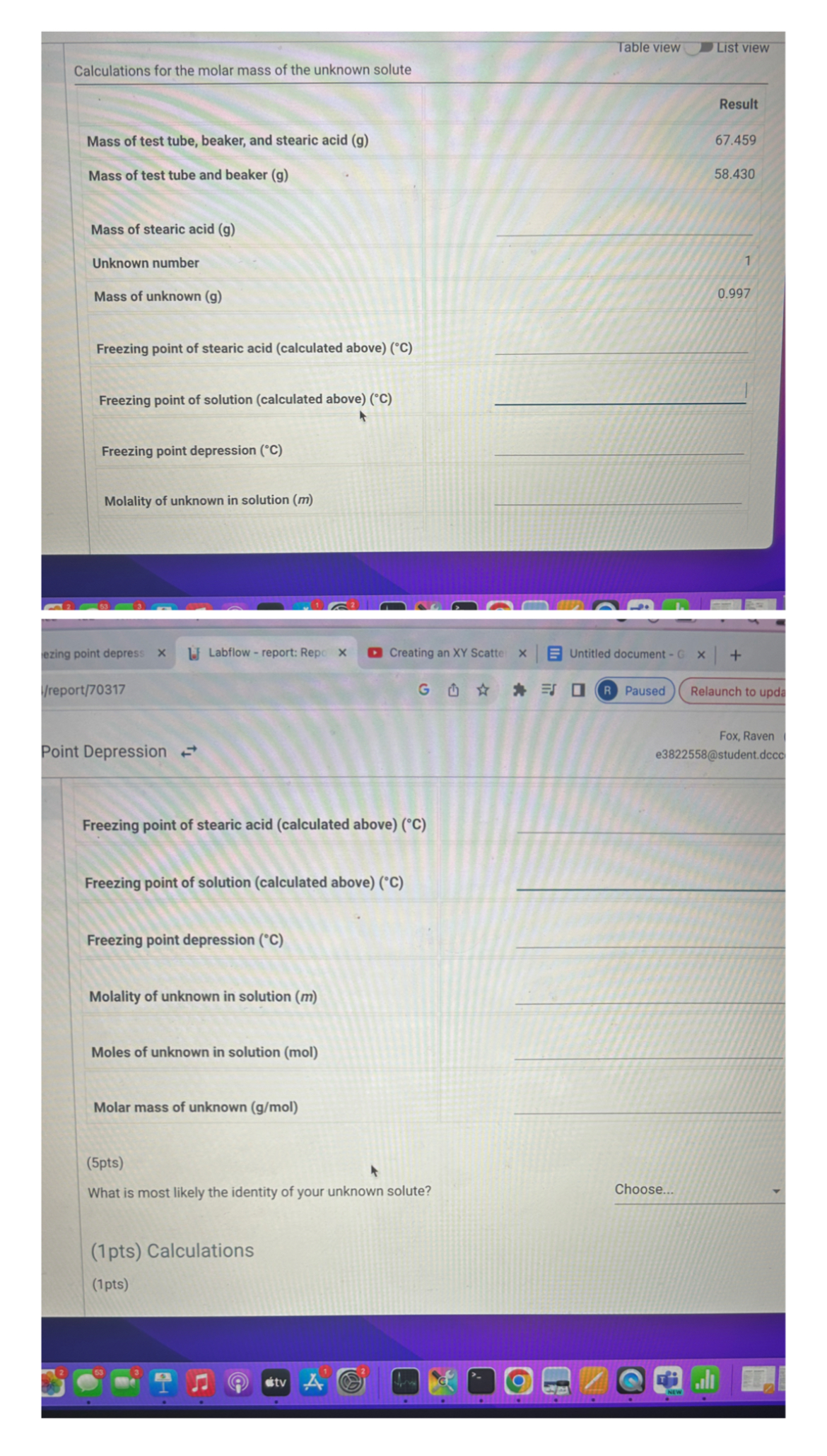
Table view List view Calculations for the molar mass of the unknown solute Result Mass of test tube, beaker, and stearic acid ( g )
Table view
List view
Calculations for the molar mass of the unknown solute
Result
Mass of test tube, beaker, and stearic acid g
Mass of test tube and beaker g
Mass of stearic acid g
Unknown number
Mass of unknown
Freezing point of stearic acid calculated above
Freezing point of solution calculated above
Freezing point depression
Molality of unknown in solution m
ezing point depress
Labflow report: Repc
Creating an XY Scattc
Untitled document
report
Paused
Relaunch to upd:
Fox, Raven
Point Depression
e@student.dccc
Freezing point of stearic acid calculated above
Freezing point of solution calculated above
Freezing point depression
Molality of unknown in solution
Moles of unknown in solution mol
Molar mass of unknown
pts
What is most likely the identity of your unknown solute?
Choose..
pts Calculations
pts
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


