Answered step by step
Verified Expert Solution
Question
1 Approved Answer
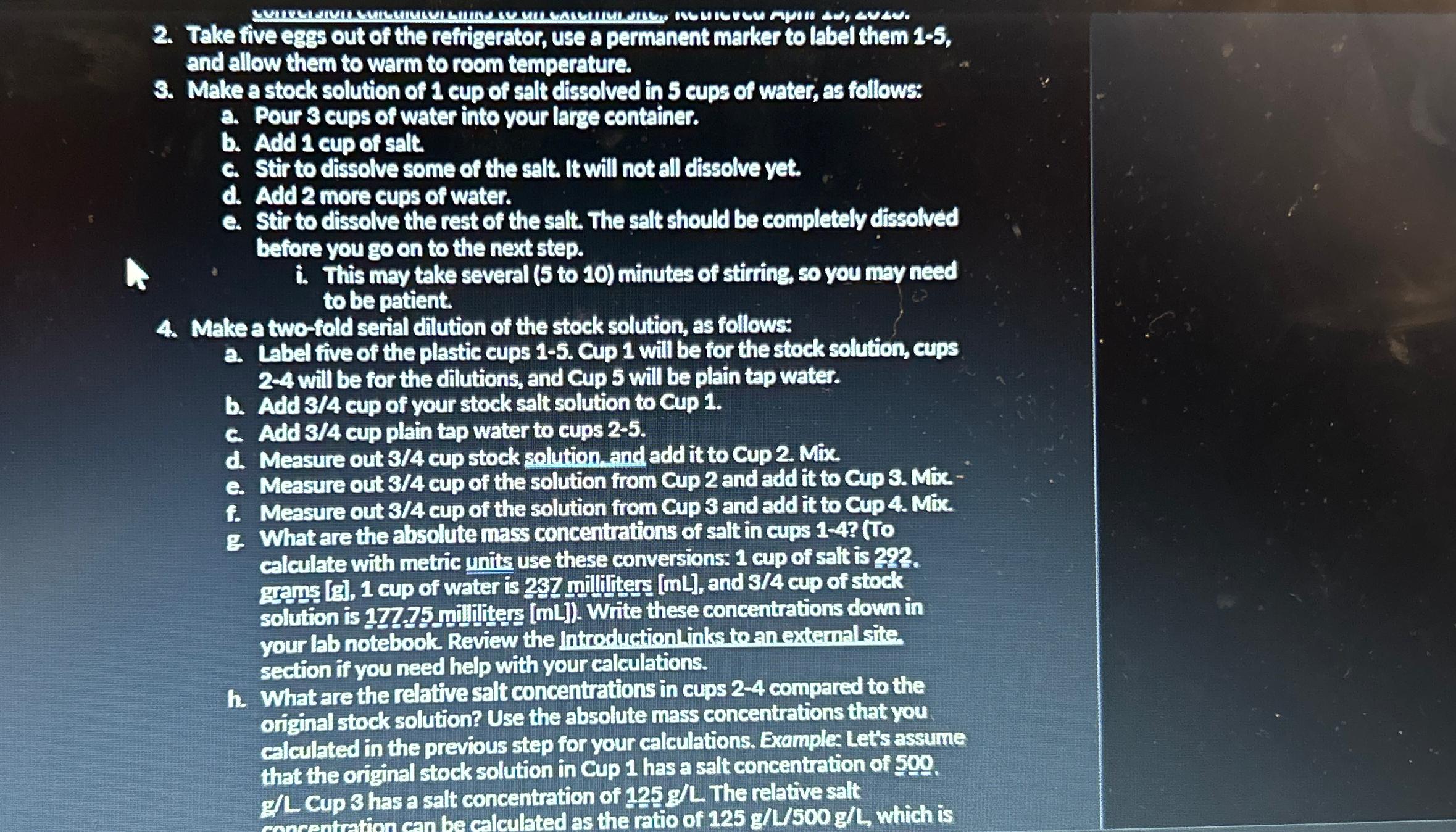
Take five eggs out of the refrigerator, use a permanent marker to label them 1 - 5 , and allow them to warm to room
Take five eggs out of the refrigerator, use a permanent marker to label them and allow them to warm to room temperature.
Make a stock solution of cup of salt dissolved in cups of water, as follows:
a Pour cups of water into your large container.
b Add cup of salt.
c Stir to dissolve some of the salt. It will not all dissolve yet.
d Add more cups of water.
e Stir to dissolve the rest of the salt. The salt should be completely dissolved before you go on to the next step.
i This may take several to minutes of stirring so you may need to be patient.
Make twofold serial dilution of the stock solution, as follows:
a Label five of the plastic cups Cup will be for the stock solution, cups will be for the dilutions, and Cup will be plain tap water.
b Add cup of your stock salt solution to Cup
c Add cup plain tap water to cups
d Measure out cup stock solution and add it to Cup Mix.
e Measure out cup of the solution from Cup and add it to Cup Mix.
f Measure out cup of the solution from Cup and add it to Cup Mix.
g What are the absolute mass concentrations of salt in cups T calculate with metric units use these conversions: cup of salt is grams g cup of water is milliliters and cup of stock solution is milliliters mL Write these concentrations down in your lab notebook Review the introduction links to an extemal site, section if you need help with your calculations.
h What are the relative salt concentrations in cups compared to the original stock solution? Use the absolute mass concentrations that you calculated in the previous step for your calculations. Example: Let's assume that the original stock solution in Cup has a salt concentration of GL Cup has a salt concentration of gL The relative salt concentration of compared to cup
Now, starting with cup and working your way up test an egg in each solution to see if it will float.
In which cup did the egg first float if the egg floated in more than one cup, did you notice any difference in your notebook, including the egg's number.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


