Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Take Quiz Exit If we look everywhere in space for an electron, the probability of finding the electron is 1. This idea can be
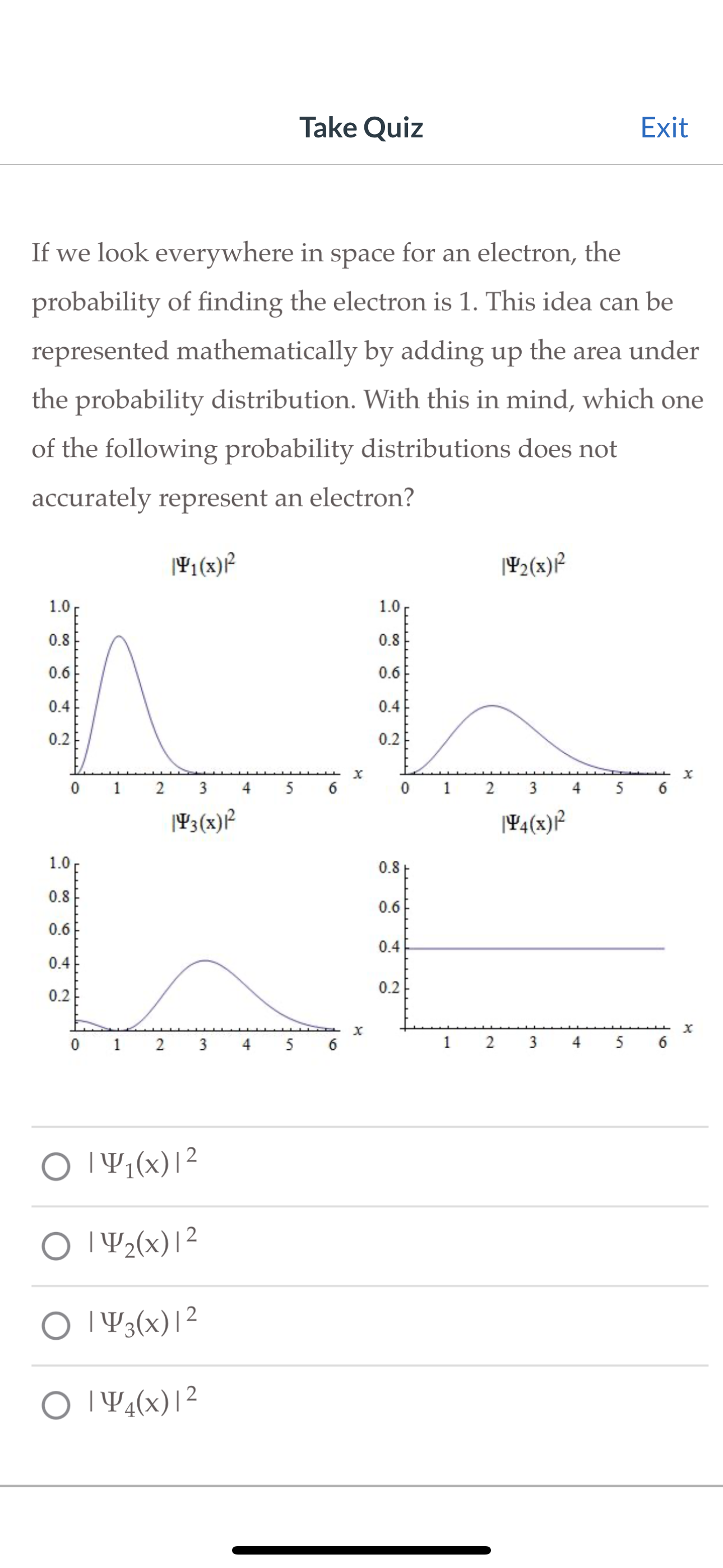
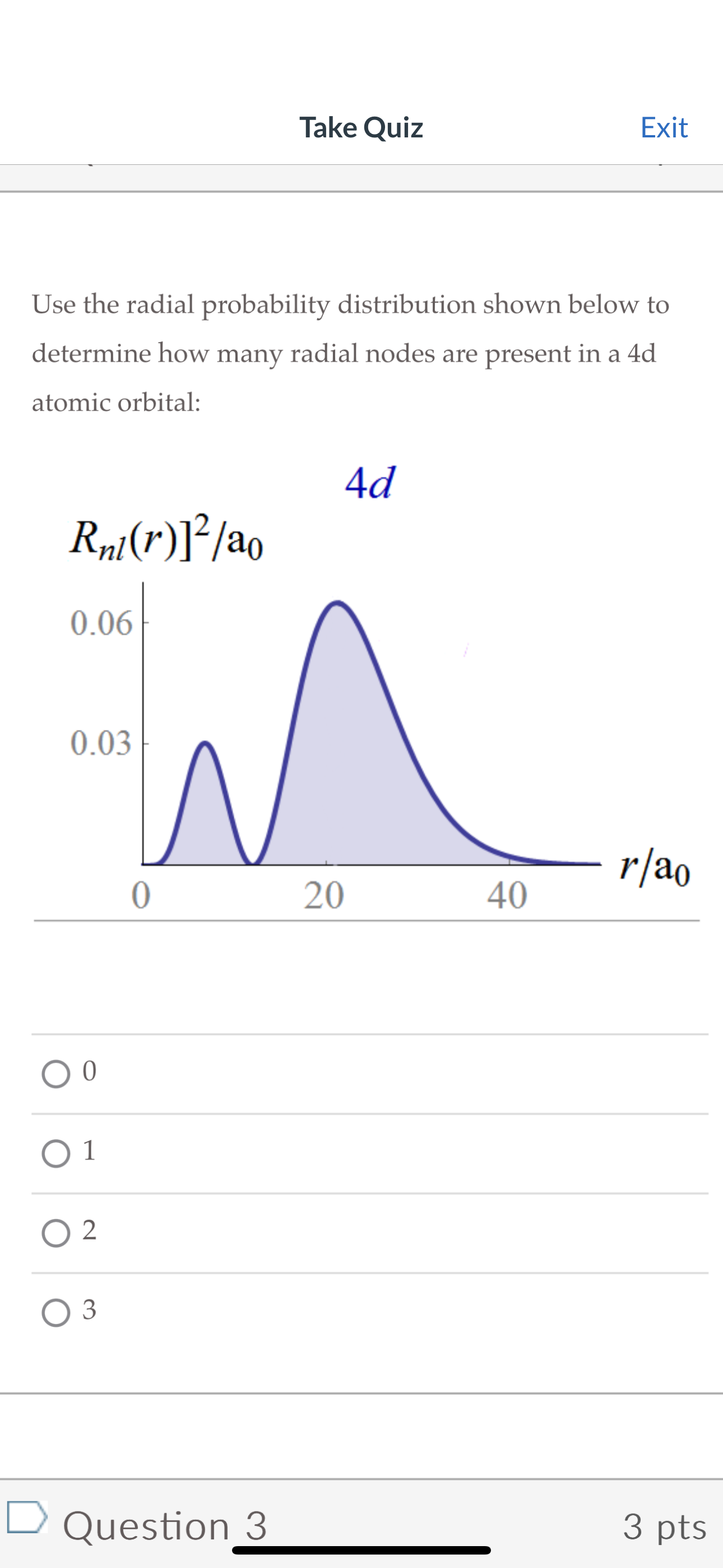
Take Quiz Exit If we look everywhere in space for an electron, the probability of finding the electron is 1. This idea can be represented mathematically by adding up the area under the probability distribution. With this in mind, which one of the following probability distributions does not accurately represent an electron? 1.0 0.8 0.6 0.4 0.2 141(x) 142(x) 1.0 0.8 0.6 0.4 0.2 1.0 0.8 0.6 0.4 0.2 0 1 2 3 5 6 143(x) * x 0 1 2 3 4 5 4(x) 0.8 0.6 0.4 0.2 x 01 2 3 4 56 1 2 | 41(x)|2 | 42(x)|2 | 3(x) | 2 O4(x)|2 3 4 5 6 X x Take Quiz Exit Use the radial probability distribution shown below to determine how many radial nodes are present in a 4d atomic orbital: Rnl(r)]/ao 0.06 0.03 0 1 2 3 4d r/ao 0 20 40 Question 3 3 pts
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


