Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Task 2: Task 3: A. At winter design conditions, a house is projected to lose heat at a rate of 60,000 Btu/h. The internal
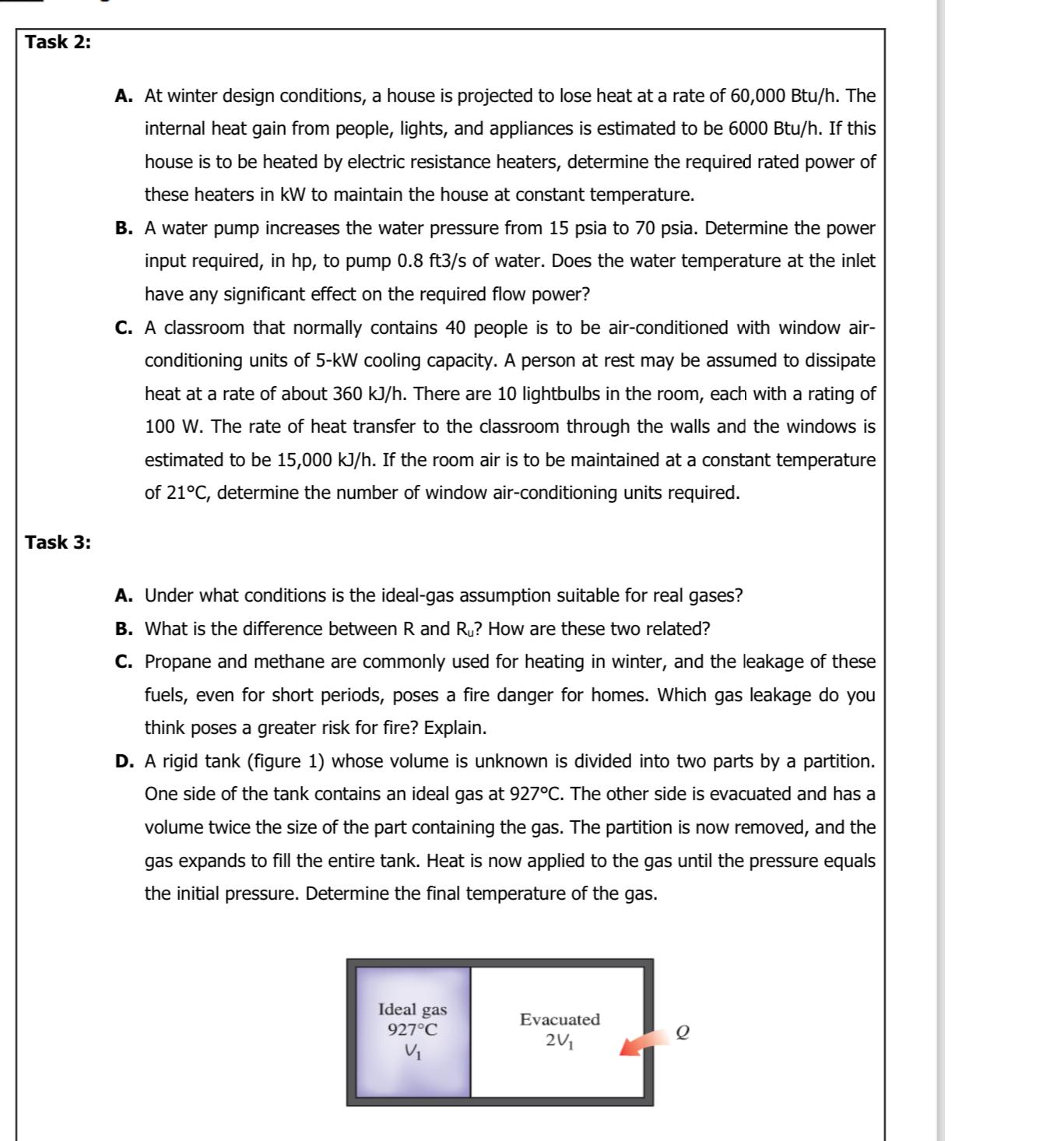
Task 2: Task 3: A. At winter design conditions, a house is projected to lose heat at a rate of 60,000 Btu/h. The internal heat gain from people, lights, and appliances is estimated to be 6000 Btu/h. If this house is to be heated by electric resistance heaters, determine the required rated power of these heaters in kW to maintain the house at constant temperature. B. A water pump increases the water pressure from 15 psia to 70 psia. Determine the power input required, in hp, to pump 0.8 ft3/s of water. Does the water temperature at the inlet have any significant effect on the required flow power? C. A classroom that normally contains 40 people is to be air-conditioned with window air- conditioning units of 5-kW cooling capacity. A person at rest may be assumed to dissipate heat at a rate of about 360 kJ/h. There are 10 lightbulbs in the room, each with a rating of 100 W. The rate of heat transfer to the classroom through the walls and the windows is estimated to be 15,000 kJ/h. If the room air is to be maintained at a constant temperature of 21C, determine the number of window air-conditioning units required. A. Under what conditions is the ideal-gas assumption suitable for real gases? B. What is the difference between R and Ru? How are these two related? C. Propane and methane are commonly used for heating in winter, and the leakage of these fuels, even for short periods, poses a fire danger for homes. Which gas leakage do you think poses a greater risk for fire? Explain. D. A rigid tank (figure 1) whose volume is unknown is divided into two parts by a partition. One side of the tank contains an ideal gas at 927C. The other side is evacuated and has a volume twice the size of the part containing the gas. The partition is now removed, and the gas expands to fill the entire tank. Heat is now applied to the gas until the pressure equals the initial pressure. Determine the final temperature of the gas. Ideal gas 927C Evacuated 2V1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


