Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Temperature { Consider the Cu-Ag phase diagram. 1000 800 Composition (at % Ag) 20 40 60 80 100 2000 Liquidus Solidus Liquid a +
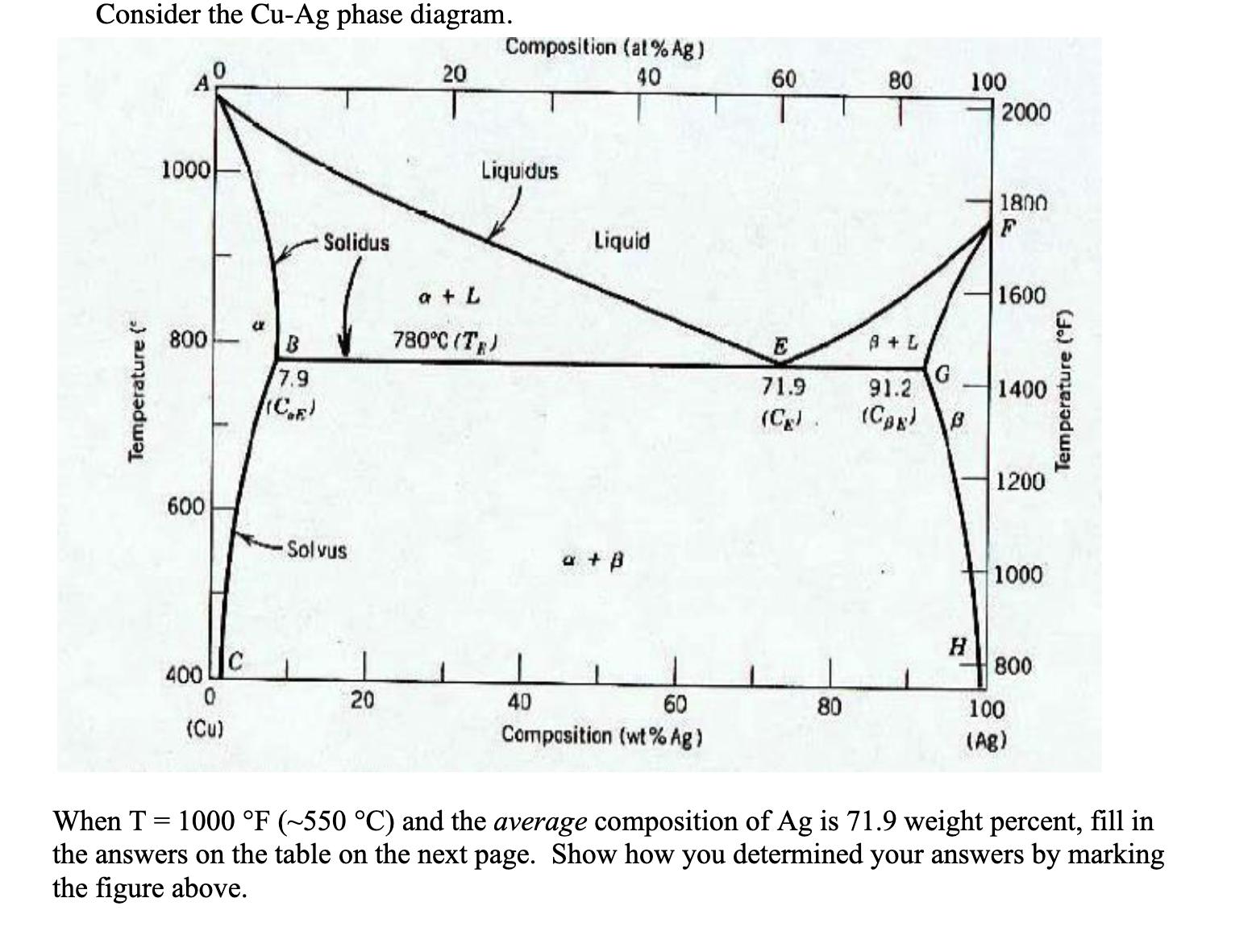
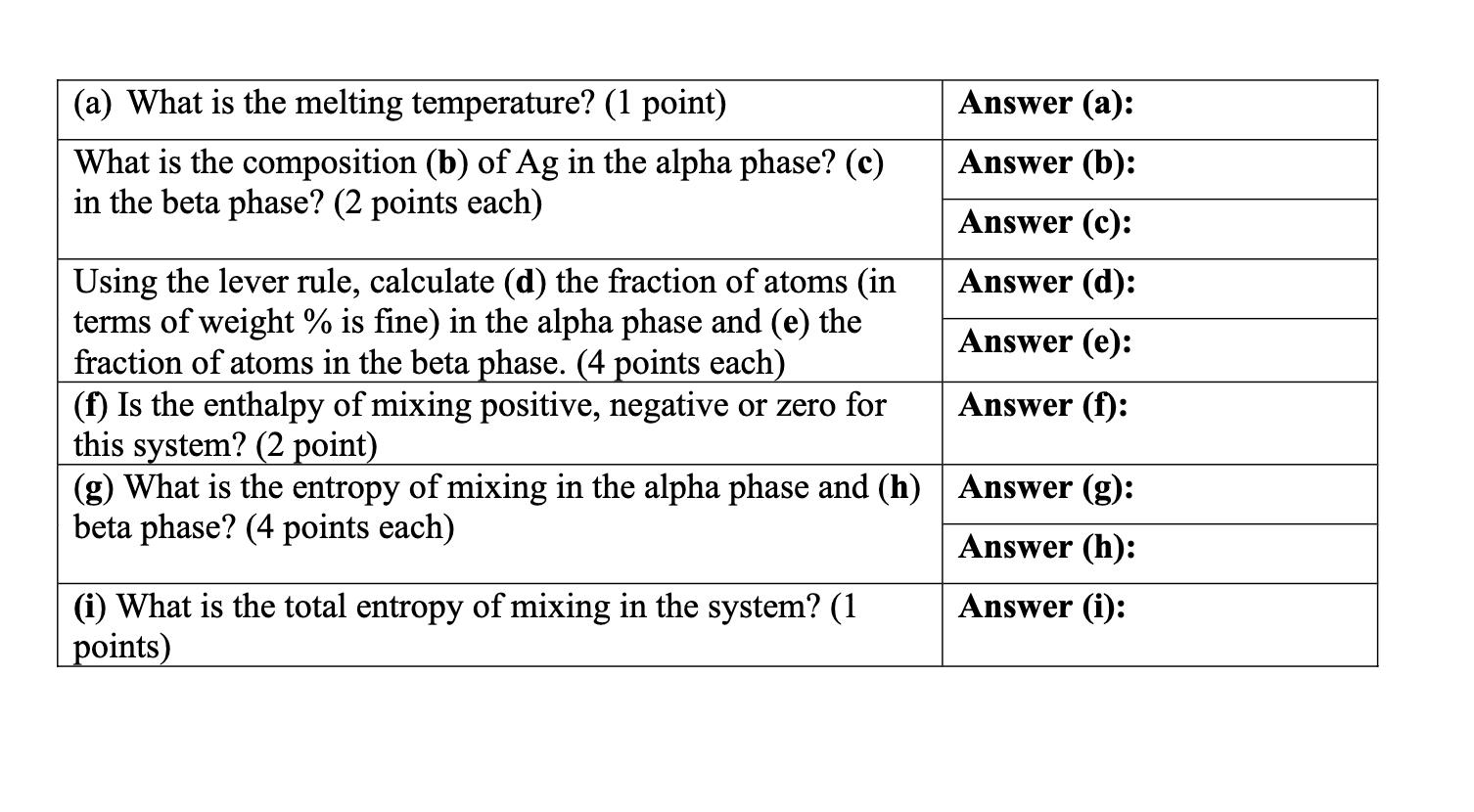
Temperature { Consider the Cu-Ag phase diagram. 1000 800 Composition (at % Ag) 20 40 60 80 100 2000 Liquidus Solidus Liquid a + L B 780C (TE) 7.9 (CF) 600 Solvus + B 1800 F 1600 E A+L 71.9 91.2 1400 (C) (CB) B 1200 1000 H 800 400 20 40 60 80 100 (Cu) Composition (wt % Ag) (Ag) Temperature (F) When T = 1000 F (~550 C) and the average composition of Ag is 71.9 weight percent, fill in the answers on the table on the next page. Show how you determined your answers by marking the figure above. (a) What is the melting temperature? (1 point) Answer (a): What is the composition (b) of Ag in the alpha phase? (c) Answer (b): in the beta phase? (2 points each) Answer (c): Using the lever rule, calculate (d) the fraction of atoms (in Answer (d): terms of weight % is fine) in the alpha phase and (e) the fraction of atoms in the beta phase. (4 points each) Answer (e): Answer (f): (f) Is the enthalpy of mixing positive, negative or zero for this system? (2 point) (g) What is the entropy of mixing in the alpha phase and (h) Answer (g): beta phase? (4 points each) (i) What is the total entropy of mixing in the system? (1 points) Answer (h): Answer (i):
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


