Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The process can be represented by two half-reactions. = anode: 2 H2O (1) O2(g) + 4H+ (aq) + 4e cathode: 4H+ (aq) + 4
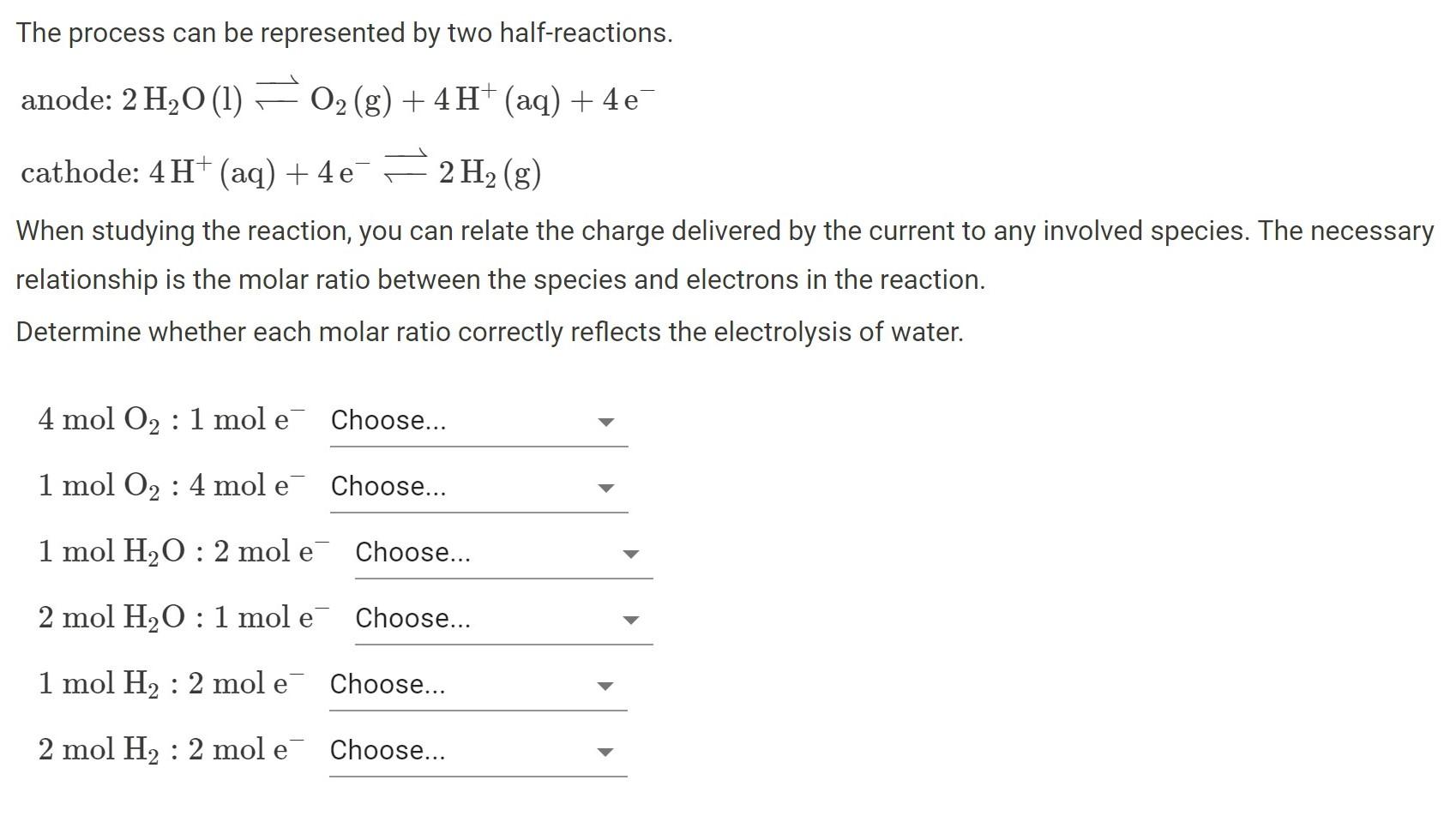
The process can be represented by two half-reactions. = anode: 2 H2O (1) O2(g) + 4H+ (aq) + 4e cathode: 4H+ (aq) + 4 e 2 H2(g) 4e When studying the reaction, you can relate the charge delivered by the current to any involved species. The necessary relationship is the molar ratio between the species and electrons in the reaction. Determine whether each molar ratio correctly reflects the electrolysis of water. 4 mol O2 : 1 mol e Choose... 1 mol O2 4 mol e Choose... 1 mol H2O: 2 mol e Choose... 2 mol H2O: 1 mol e Choose... 1 mol H2 2 mol e Choose... 2 mol H2 2 mol e Choose...
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To answer these questions lets analyze the electrolysis of water and the corresponding halfreactions ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


