Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Tfinal= 40C Tfinal= 20.68C Tfinal= 22.73 C WATER WATER EXPERIMENT 1A LIQUID/SOLID/SOLUTION MASS (9) TEMPERATURE (C) FINAL TEMPERATURE (T final in C) 100 g 100
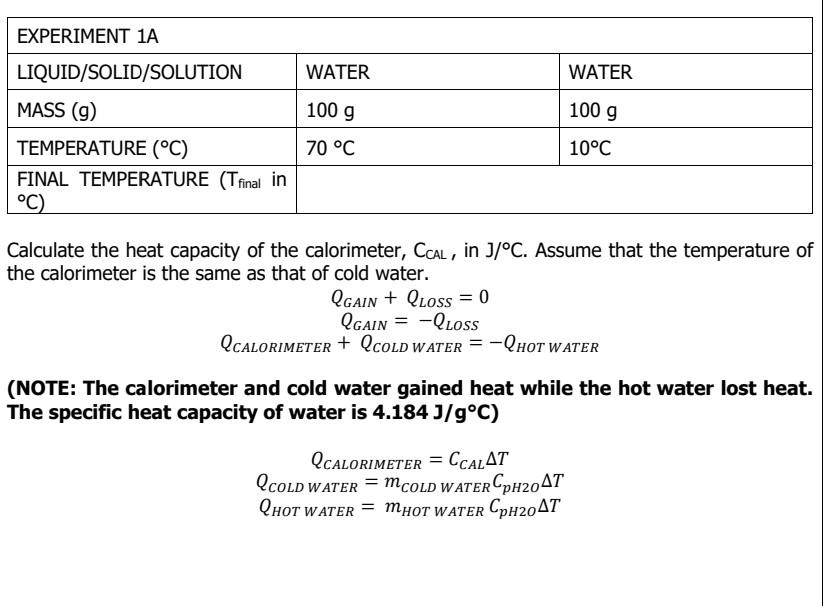
Tfinal= 40C
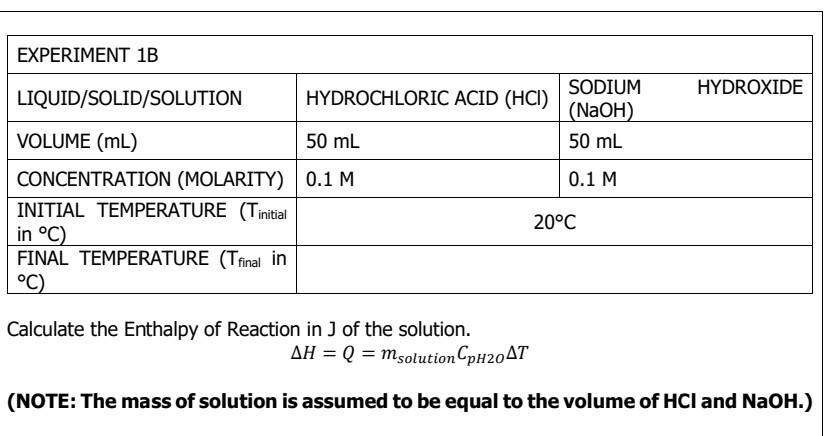
Tfinal= 20.68C
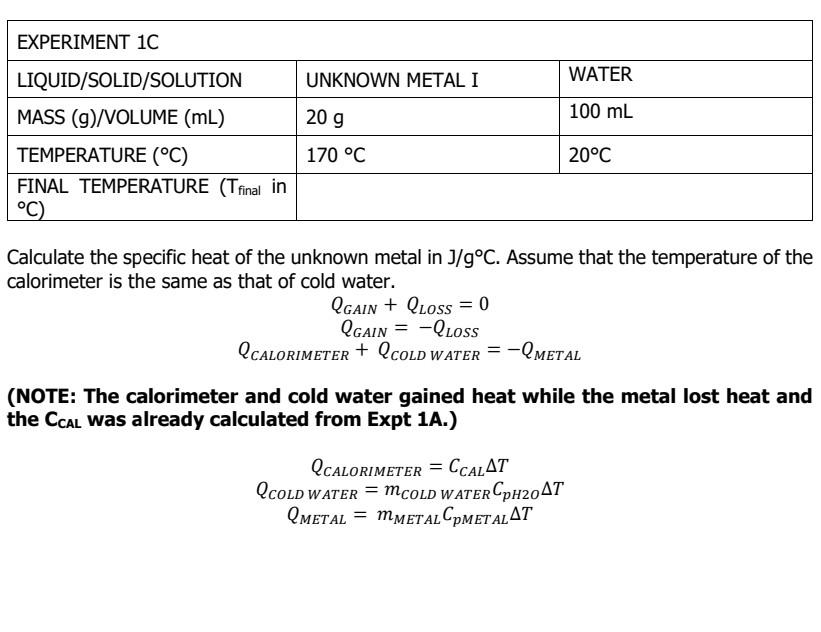
Tfinal= 22.73C

WATER WATER EXPERIMENT 1A LIQUID/SOLID/SOLUTION MASS (9) TEMPERATURE (C) FINAL TEMPERATURE (T final in C) 100 g 100 g 70 C 10C Calculate the heat capacity of the calorimeter, CCAL, in J/C. Assume that the temperature of the calorimeter is the same as that of cold water. QGAIN + Qloss = 0 Qgain = -Qloss CALORIMETER + QCOLD WATER = - HOT WATER (NOTE: The calorimeter and cold water gained heat while the hot water lost heat. The specific heat capacity of water is 4.184 J/gC) QCALORIMETER = CCALAT lcold water = MCOLD WATER CpH204T QHot water = MHOT WATER Cph204T EXPERIMENT 1B HYDROXIDE LIQUID/SOLID/SOLUTION HYDROCHLORIC ACID (HCI) SODIUM (NaOH) 50 mL 0.1 M VOLUME (mL) 50 mL CONCENTRATION (MOLARITY) 0.1 M INITIAL TEMPERATURE (Tinitial in C) FINAL TEMPERATURE (Tfinal in C) 20C Calculate the Enthalpy of Reaction in J of the solution. AH = Q = m solution CpH204T (NOTE: The mass of solution is assumed to be equal to the volume of HCl and NaOH.) UNKNOWN METAL I WATER EXPERIMENT 1C LIQUID/SOLID/SOLUTION MASS (9)/VOLUME (mL) TEMPERATURE (C) FINAL TEMPERATURE (Tfinal in C) 20 g 100 mL 170 C 20C Calculate the specific heat of the unknown metal in J/gC. Assume that the temperature of the calorimeter is the same as that of cold water. QGAIN + Qloss = 0 Qgain = -Qloss QCALORIMETER + QCOLD WATER = -Q METAL (NOTE: The calorimeter and cold water gained heat while the metal lost heat and the CCAL was already calculated from Expt 1A.) QCALORIMETER = CCALAT QCOLD WATER = MCOLD WATERCpH20AT QMETAL = MMETAL CpMETALAT 2. A piece of metal weighing 59.047g was heated to 100C and then put in a 100mL water at 23.7C. The metal and the water were allowed to reach the equilibrium temperature of 27.8C. Calculate the specific heat of the metal when the calorimeter constant is 100 J/C
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


