Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Thank you Concentrations of lodine Species Question 14 (1 point) A student determined the average concentration of total reducible iodine in the aqueous phase from
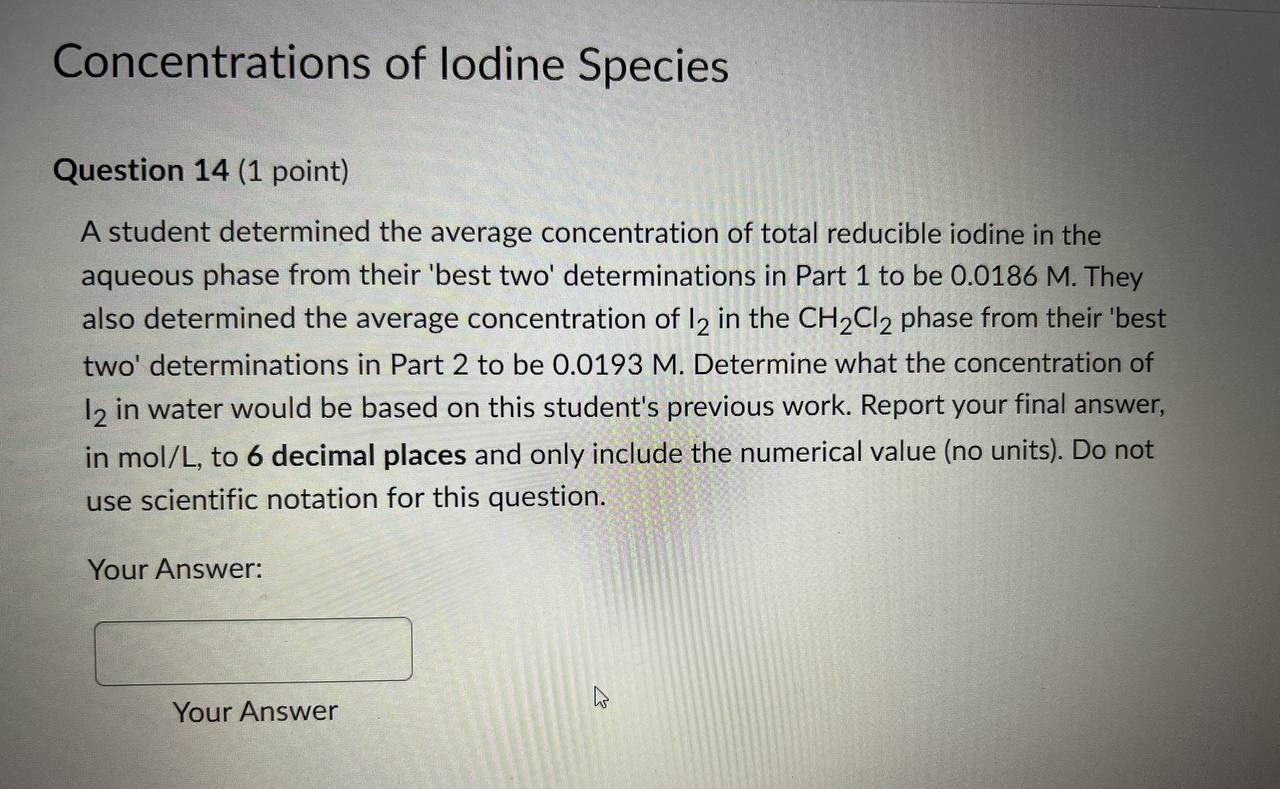

Thank you
Concentrations of lodine Species Question 14 (1 point) A student determined the average concentration of total reducible iodine in the aqueous phase from their 'best two' determinations in Part 1 to be 0.0186M. They also determined the average concentration of I2 in the CH2Cl2 phase from their 'best two' determinations in Part 2 to be 0.0193M. Determine what the concentration of I2 in water would be based on this student's previous work. Report your final answer, in mol/L, to 6 decimal places and only include the numerical value (no units). Do not use scientific notation for this question. Your Answer: Your Answer Question 15 (1 point) A student determined the concentration of total reducible iodine in the aqueous phase based on the average of their 'best two' determinations in Part 1 to be 0.0179 M. They also determined the concentration of I2 in the CH2Cl2 phase based on the average of their 'best two' determinations in Part 2 to be 0.0196M. Determine what the concentration of I3 - in water would be based on this student's previous work. Report your final answer, in mol/L, to 4 decimal places and only include the numerical value (no units). Do not use scientific notation for this question. Your Answer: YourStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


