Answered step by step
Verified Expert Solution
Question
1 Approved Answer
thank you for answering Which of the following statements is true about the Lewis structure of an ionic compound and the use of square brackets?
thank you for answering 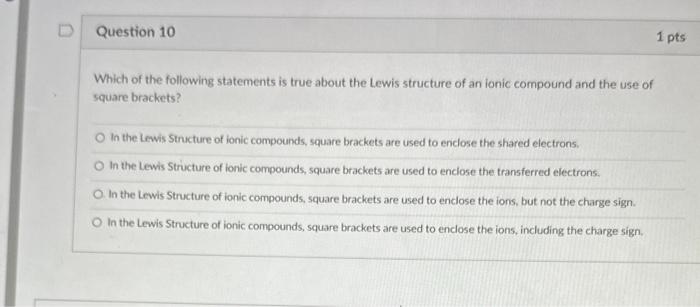

Which of the following statements is true about the Lewis structure of an ionic compound and the use of square brackets? In the Lewis Structure of ionic compounds, square brackets are used to enclose the shared electrons. In the Lewis Structure of ionic compounds, square brackets are used to enciose the transferred electrons. In the Lewis Structure of ionic compounds, square brackets are used to enclose the ions, but not the charge sign. In the Lewis Structure of ionic compounds, square brackets are used to enclose the ions, including the charge sign. When writing a Lewis structure, which of the following is the correct order of steps? Determine the number of valence electrons, determine the outer atoms, place the central atom, and add electrons to satisfy the octet rule. Determine the number of valence electrons, determine the central atom, place the outer atoms around the central atom. and add electrons to satisfy the octet rule. Determine the number of valence electrons, determine the central atom, place the outer atoms around the central atom, and add protons to satisfy the octet rule. Determine the number of electrons, determine the central atom, place the outer atoms around the central atom, and add protons to satisfy the octet rule 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


