Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Thank you for helping :) 1. A coal has the following composition by weight C = 75%,O= 23%, 5=1%, N=1% and ash = 5%. Net
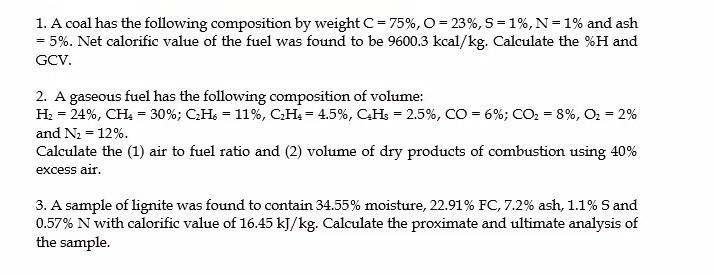
Thank you for helping :)
1. A coal has the following composition by weight C = 75%,O= 23%, 5=1%, N=1% and ash = 5%. Net calorific value of the fuel was found to be 9600.3 kcal/kg. Calculate the %H and GCV. 2. A gaseous fuel has the following composition of volume: H2 = 24%, CH: = 30%; CHs = 11%, CH: = 4.5%, CHs = 2.5%, CO = 6%; CO2 = 8%, O2 = 2% and N2 = 12%. Calculate the (1) air to fuel ratio and (2) volume of dry products of combustion using 40% excess air. 3. A sample of lignite was found to contain 34.55% moisture, 22.91% FC, 7.2% ash, 1.1% Sand 0.57% N with calorific value of 16.45 kJ/kg. Calculate the proximate and ultimate analysis of the sampleStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


