Question
Thank you for your help! 1. Calculate the volume of water delivered into the beaker. To find this, subtract your recorded pipette reading from 5.00
Thank you for your help!
1. Calculate the volume of water delivered into the beaker. To find this, subtract your recorded pipette reading from 5.00 mL (5.00 mL your pipette reading mL = volume of water delivered).
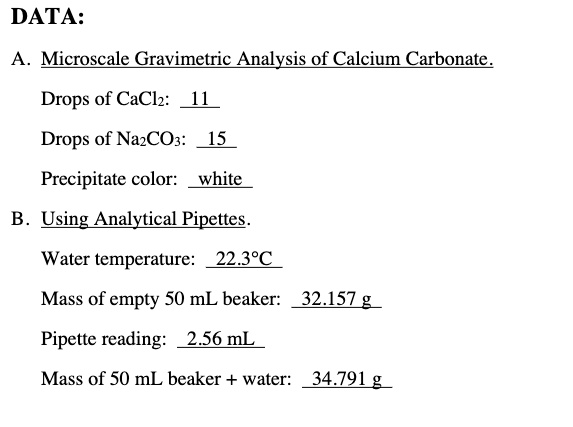
I got 5-2.56mL= 2.44mL for this
2. What mass of water did the pipette deliver?
I think this is question 1 answer? I'm not sure if it's another calculation.
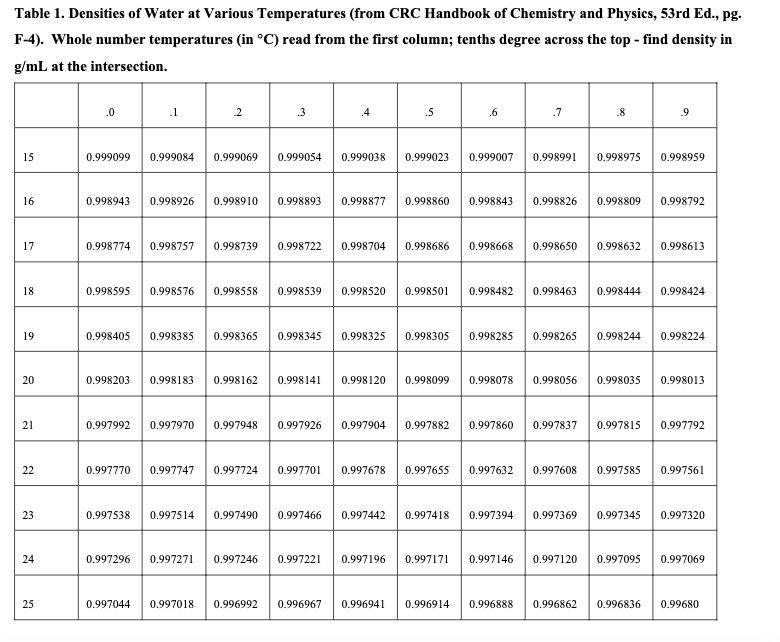
3. Use water temperature, water mass, and density (see Table 1) to calculate the volume of water delivered based on your mass measurement. Show your work in the lab notebook.
I'm not sure what equation to use here with the info from the table.
4. The calculated pipette volume should be within 0.02 mL of the recorded volume. Is your value within the error limit?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


