Answered step by step
Verified Expert Solution
Question
1 Approved Answer
thank you for your help Which of the following statements is true regarding the octet rule in Lewis structures? Atoms with more than eight valence
thank you for your help 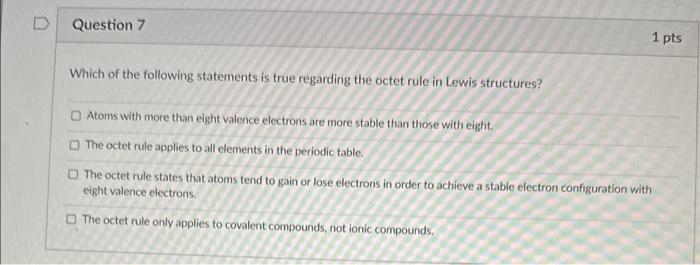
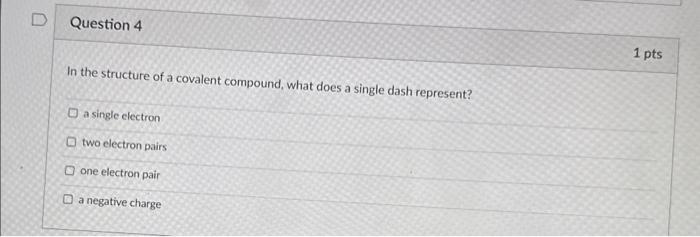
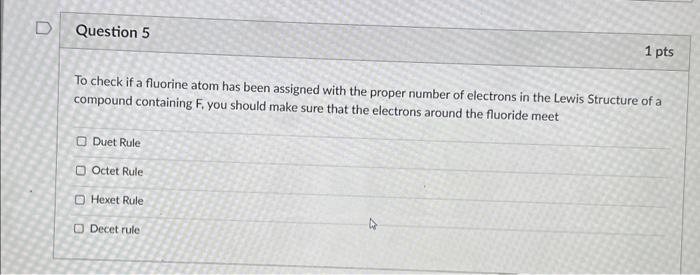
Which of the following statements is true regarding the octet rule in Lewis structures? Atoms with more than eight valence electrons are more stable than those with eight. The octet rule applies to all elements in the periodic table. The octet rule states that atoms tend to gain or lose electrons in order to achieve a stable electron configuration with eight valence electrons. The octet rule only applies to covalent compounds, not ionic compounds. In the structure of a covalent compound, what does a single dash represent? a single electron two electron pairs one electron pair a negative charge To check if a fluorine atom has been assigned with the proper number of electrons in the Lewis Structure of a compound containing F, you should make sure that the electrons around the fluoride meet Duet Rule Octet Rule Hexet Rule Decet rule 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


