Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Thank you in advance for your help Oxidation number -Jan 18 & 19, 2021 1 9 P=. RbO, O= Determine the oxidation number of each
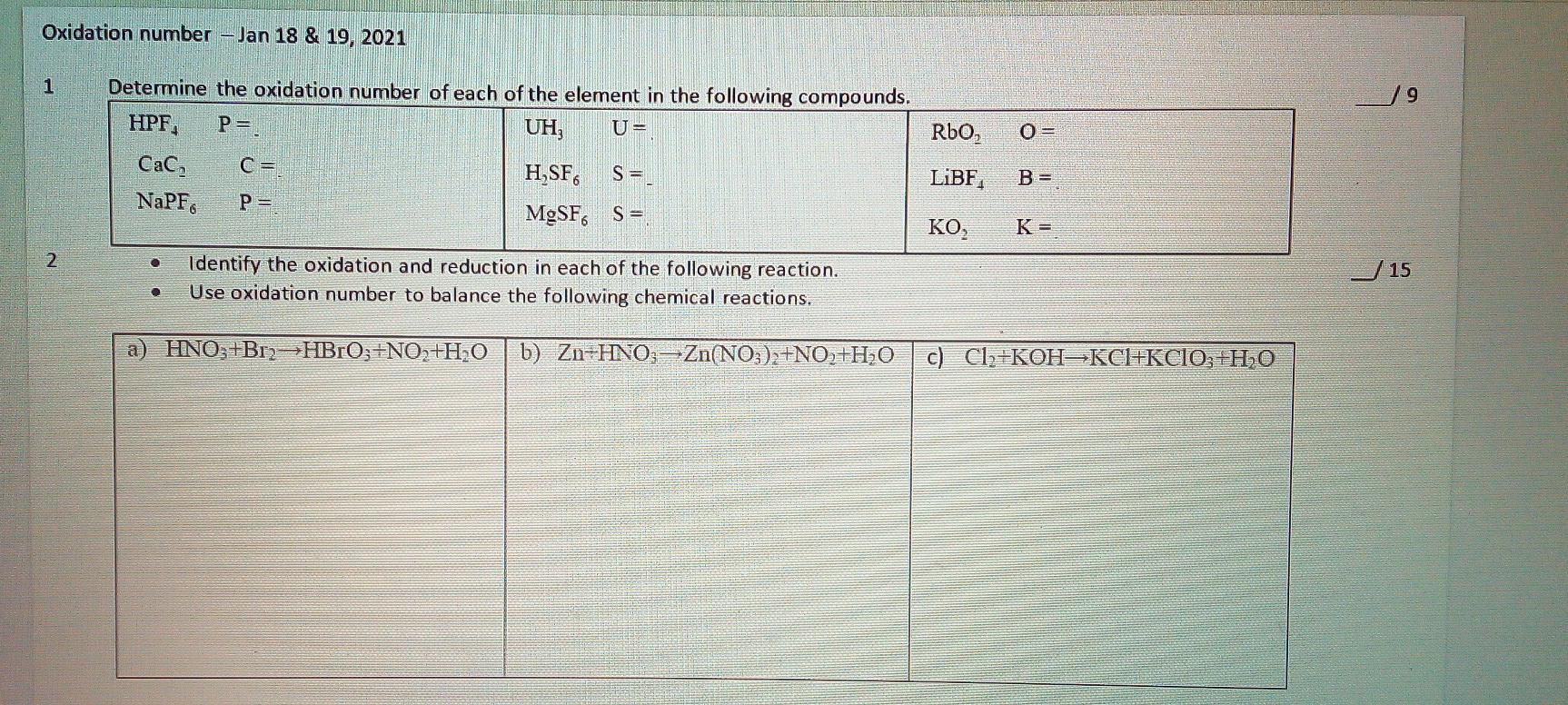
Thank you in advance for your help
Oxidation number -Jan 18 & 19, 2021 1 9 P=. RbO, O= Determine the oxidation number of each of the element in the following compounds. HPF. UH, U= , C= H SF S = NaPF P= MgSF. Sa LiBF B = KO K = 2 15 Identify the oxidation and reduction in each of the following reaction. Use oxidation number to balance the following chemical reactions. a) HNO3+B12>HBrO;+NO2+H2O b) Zn HNO3 Zn(NO3)2+NO+H2O c) Cl2+KOH-KCI+KCIO; +H2OStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


