Answered step by step
Verified Expert Solution
Question
1 Approved Answer
thank you in advance Q5. (7 marks total) Austenitic steels can be formed by the interstitial solution of alloying elements into the tetrahedral sites of
thank you in advance 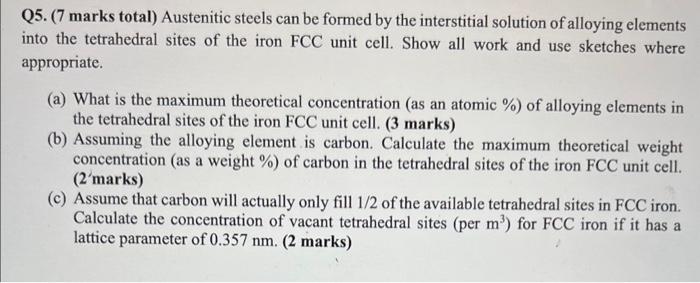
Q5. (7 marks total) Austenitic steels can be formed by the interstitial solution of alloying elements into the tetrahedral sites of the iron FCC unit cell. Show all work and use sketches where appropriate. (a) What is the maximum theoretical concentration (as an atomic \%) of alloying elements in the tetrahedral sites of the iron FCC unit cell. (3 marks) (b) Assuming the alloying element is carbon. Calculate the maximum theoretical weight concentration (as a weight \%) of carbon in the tetrahedral sites of the iron FCC unit cell. (2'marks) (c) Assume that carbon will actually only fill 1/2 of the available tetrahedral sites in FCC iron. Calculate the concentration of vacant tetrahedral sites (per m3 ) for FCC iron if it has a lattice parameter of 0.357nm. ( 2 marks) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


