Answered step by step
Verified Expert Solution
Question
1 Approved Answer
thank you so much! Q3(25%). An SOFC operates at 900C on a gaseous fuel ( 20%CO;80%H2, by volume). At this temperature any H2O formed is
thank you so much! 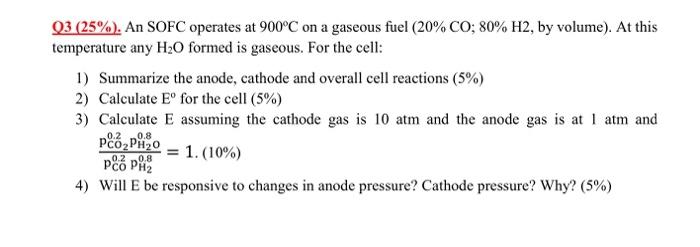
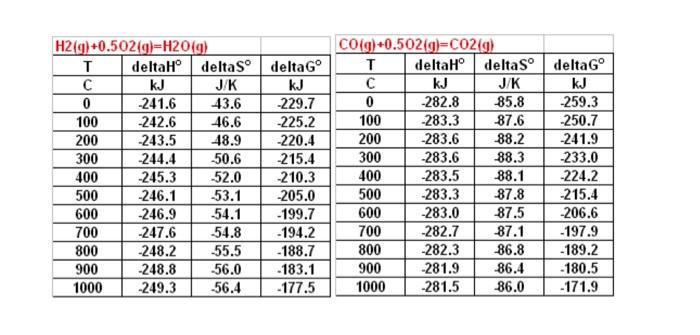
Q3(25%). An SOFC operates at 900C on a gaseous fuel ( 20%CO;80%H2, by volume). At this temperature any H2O formed is gaseous. For the cell: 1) Summarize the anode, cathode and overall cell reactions ( 5%) 2) Calculate E for the cell (5%) 3) Calculate E assuming the cathode gas is 10atm and the anode gas is at 1atm and pCO0.2pH20.8pCO20.2pH2O0.8=1.(10%) 4) Will E be responsive to changes in anode pressure? Cathode pressure? Why? (5\%) \begin{tabular}{|c|c|c|c|} \hline \multicolumn{2}{|c|}{CO(g)+0.502(g)=CO(g)} & \\ \hline T & deltaH & deltaS & deltaG \\ \hline C & kJ & J/K & kJ \\ \hline 0 & -282.8 & -85.8 & -259.3 \\ \hline 100 & -283.3 & -87.6 & -250.7 \\ \hline 200 & -283.6 & -88.2 & -241.9 \\ \hline 300 & -283.6 & -88.3 & -233.0 \\ \hline 400 & -283.5 & -88.1 & -224.2 \\ \hline 500 & -283.3 & -87.8 & -215.4 \\ \hline 600 & -283.0 & -87.5 & -206.6 \\ \hline 700 & -282.7 & -87.1 & -197.9 \\ \hline 800 & -282.3 & -86.8 & -189.2 \\ \hline 900 & -281.9 & -86.4 & -180.5 \\ \hline 1000 & -281.5 & -86.0 & -171.9 \\ \hline \end{tabular} 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


