Answered step by step
Verified Expert Solution
Question
1 Approved Answer
That's the question and also the hints for doing Q2. What is the weight of CO2 contained in cylinder with a capacity of 0.1 m
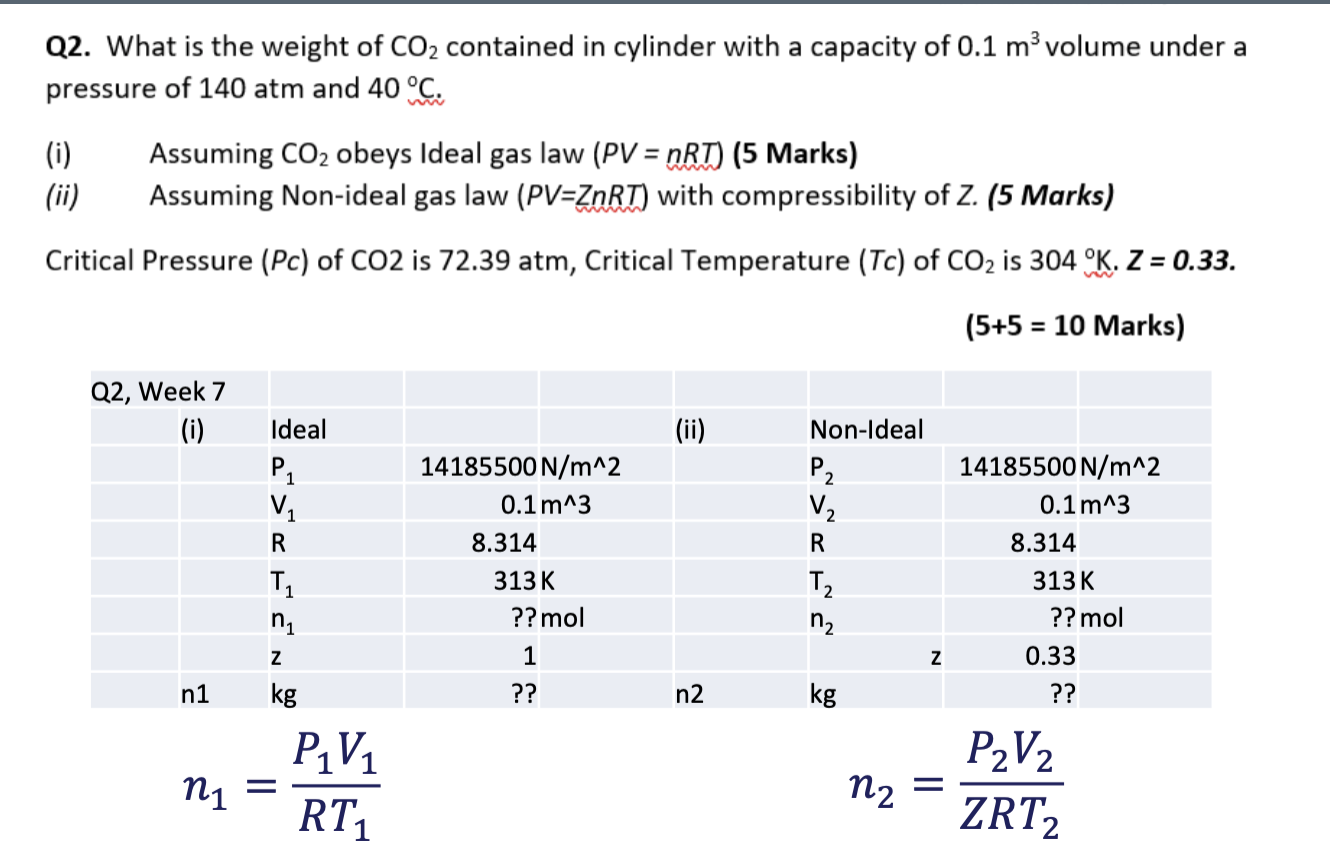
That's the question and also the hints for doing
Q2. What is the weight of CO2 contained in cylinder with a capacity of 0.1 m volume under a pressure of 140 atm and 40C. (i) (ii) Assuming CO2 obeys Ideal gas law (PV = nRT) (5 Marks) Assuming Non-ideal gas law (PV=ZnRT) with compressibility of Z. (5 Marks) Critical Pressure (Pc) of CO2 is 72.39 atm, Critical Temperature (Tc) of CO2 is 304 K. Z = 0.33. = (5+5 = 10 Marks) = Q2, Week 7 (i) (ii) Ideal PL V R T ni 14185500 N/m^2 0.1 m^3 8.314 313K ?? mol Non-Ideal P2 V R T n Z kg 14185500 N/m^2 0.1 m^3 8.314 313K ?? mol 0.33 ?? z 1 n1 kg ?? n2 P1V1 ni = n2 P2V2 ZRT2 RT1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


