Question
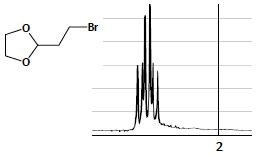
The 1 H-NMR spectrum of 2-(2-bromoethyl)-1,3-dioxolane (at o right) shows a 2H signal near 2 ppm (far right). (a) What would you call this pattern?
The 1 H-NMR spectrum of 2-(2-bromoethyl)-1,3-dioxolane (at o right) shows a 2H signal near 2 ppm (far right).
(a) What would you call this pattern?
(b) Which hydrogen(s) gave rise to this signal?
(c) Draw a splitting tree corresponding to this pattern.
-Br
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Circled CH2 group protons gives a quartet signal near 20 ppm ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
11th edition
1118133579, 978-1118133576
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



