Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The 3 reactions below allow the production of phenol from benzene: The pass conversions for these reactions are 90%, 90%, and 100%, respectively. In the
The 3 reactions below allow the production of phenol from benzene:
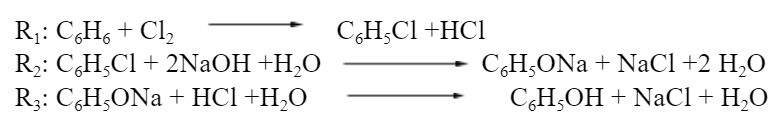 The pass conversions for these reactions are 90%, 90%, and 100%, respectively. In the first two, only reagents are allowed in the feeds. In the third, in addition to the reactants, NaCl, C6H5Cl and NaOH are allowed. Propose 3 alternative flowcharts for this reaction system.
The pass conversions for these reactions are 90%, 90%, and 100%, respectively. In the first two, only reagents are allowed in the feeds. In the third, in addition to the reactants, NaCl, C6H5Cl and NaOH are allowed. Propose 3 alternative flowcharts for this reaction system.
Question about process synthesis and optimization.
R1: C6H6 + Cl2 CH,Cl +HCI R2: C6H3Cl + 2NaOH +H2O C6H ONa+ NaCl +2 H2O R3: C6H,ONa + HCl +H2O C6H,OH + NaCl + H2OStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


