The above is a diagram from the textbook, showing the appearance of red blood cells in three different solutions. The three names of the
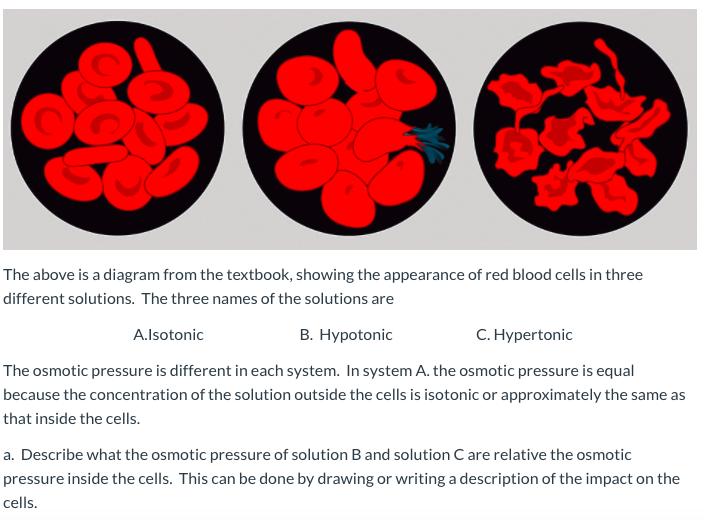
The above is a diagram from the textbook, showing the appearance of red blood cells in three different solutions. The three names of the solutions are . otonic . ertonic A.lsotonic The osmotic pressure is different in each system. In system A. the osmotic pressure is equal because the concentration of the solution outside the cells is isotonic or approximately the same as that inside the cells. a. Describe what the osmotic pressure of solution B and solution C are relative the osmotic pressure inside the cells. This can be done by drawing or writing a description of the impact on the cells.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


