Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The acid-catalyzed reaction of 4,4-dimethylcyclohexa-2,5-dien-1-one resulted in the formation of a compound, the NMR data of which are given below. For N: 1HNMR(300MHz,CDCl3);=6.95(d,J=8.0Hz,1H),6.61(d,J=2.8H,ll),6.57 (dd,J=8.0,2.8Hz,1H),5.39 (bs,
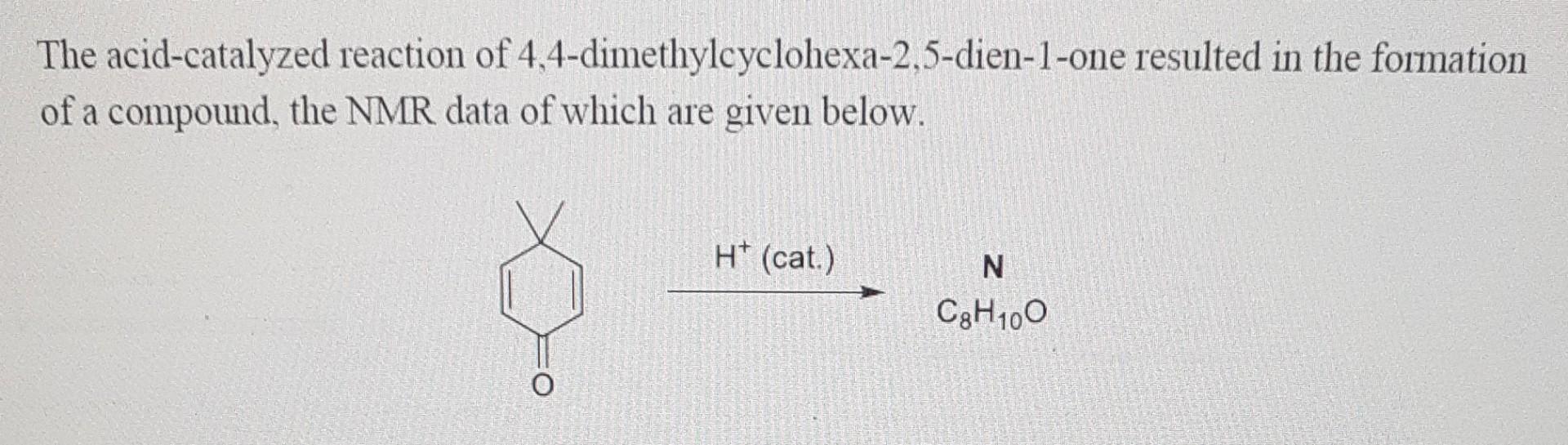

The acid-catalyzed reaction of 4,4-dimethylcyclohexa-2,5-dien-1-one resulted in the formation of a compound, the NMR data of which are given below. For N: 1HNMR(300MHz,CDCl3);=6.95(d,J=8.0Hz,1H),6.61(d,J=2.8H,ll),6.57 (dd,J=8.0,2.8Hz,1H),5.39 (bs, 1H),2.16(s,3H),2.14(s,3H),13CNMK(109MH/,CDOl3); 153.4,137.9,130.4,128.6,116.6,112.3,19.8,18.7. 4.4. Find the structure of product N and propose a plausible mechanism
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


