Answered step by step
Verified Expert Solution
Question
1 Approved Answer
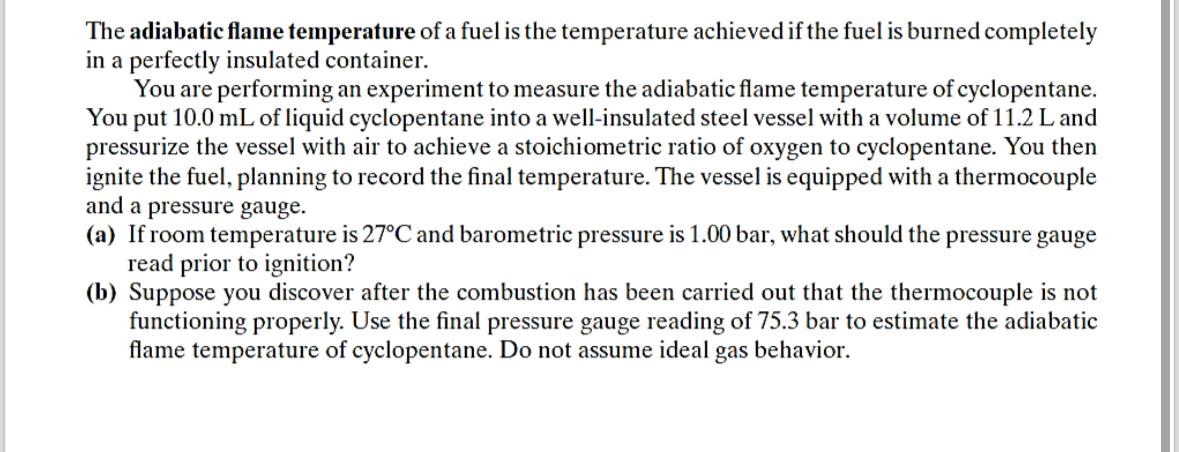
The adiabatic flame temperature of a fuel is the temperature achieved if the fuel is burned completely in a perfectly insulated container. You are performing
The adiabatic flame temperature of a fuel is the temperature achieved if the fuel is burned completely in a perfectly insulated container.
You are performing an experiment to measure the adiabatic flame temperature of cyclopentane. You put of liquid cyclopentane into a wellinsulated steel vessel with a volume of and pressurize the vessel with air to achieve a stoichiometric ratio of oxygen to cyclopentane. You then ignite the fuel, planning to record the final temperature. The vessel is equipped with a thermocouple and a pressure gauge.
a If room temperature is and barometric pressure is what should the pressure gauge read prior to ignition?
b Suppose you discover after the combustion has been carried out that the thermocouple is not functioning properly. Use the final pressure gauge reading of bar to estimate the adiabatic flame temperature of cyclopentane. Do not assume ideal gas behavior.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


