Question
The alcohols 1-butanol and 2-butanol have the same molecular weight, but 2-butanol has a higher vapor pressure due to the secondary alcohol structure. This problem
The alcohols 1-butanol and 2-butanol have the same molecular weight, but 2-butanol has a higher vapor pressure due to the secondary alcohol structure. This problem considers the bubble temperature for a mixture of the two alcohols with a liquid composition of 30 mol% 1-butanol at a pressure of 500 mm Hg. Use Raoults law for any calculations. Vapor pressuresf or two temperatures are tabulated below.
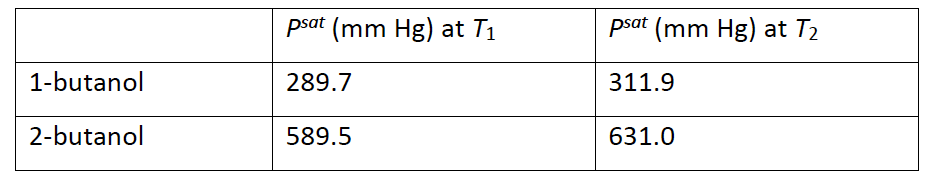
a. Evaluate both options to decide which is the bubble temperature (within expected tolerance). Indicate which column represents the bubble temperature, T1 or T2 and why you selected that temperature.
b.For the temperature T1 or T2 that is not the bubble temperature, is the temperature above or below the bubble temperature and how do you know?
c.What are the VLE K-ratios for the two components at the bubble point temperature?
\begin{tabular}{|l|l|l|} \hline & Psat(mmHg) at T1 & Psat(mmHg) at T2 \\ \hline 1-butanol & 289.7 & 311.9 \\ \hline 2-butanol & 589.5 & 631.0 \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


