Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The ammonia synthesis reaction discussed in class is too slow at low temperature, but the equilibrium limits conversion at high temperatures. Explore impact of
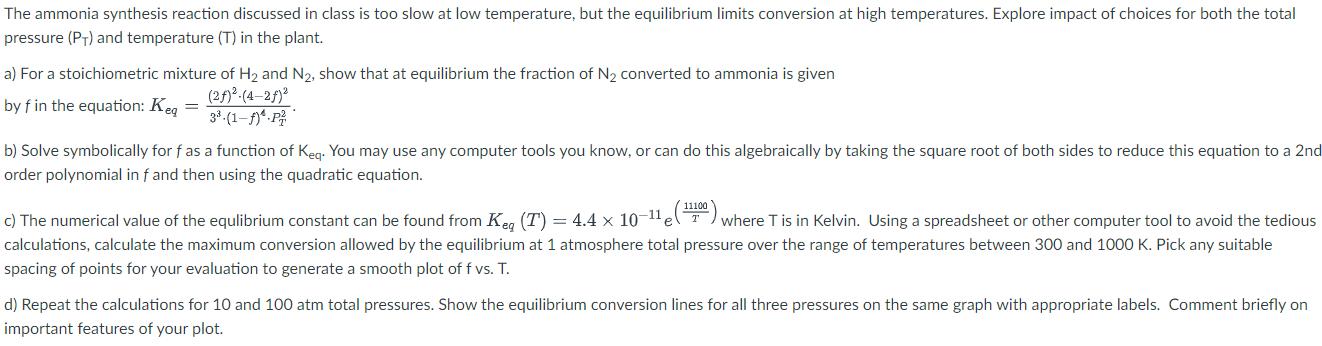
The ammonia synthesis reaction discussed in class is too slow at low temperature, but the equilibrium limits conversion at high temperatures. Explore impact of choices for both the total pressure (PT) and temperature (T) in the plant. a) For a stoichiometric mixture of H2 and N2, show that at equilibrium the fraction of N2 converted to ammonia is given by f in the equation: Keq (2)(4-2) 3.(1-f)*.P b) Solve symbolically for f as a function of Keq. You may use any computer tools you know, or can do this algebraically by taking the square root of both sides to reduce this equation to a 2nd order polynomial in f and then using the quadratic equation. c) The numerical value of the equlibrium constant can be found from Keg (T) = 4.4 1011 (1100) where T is in Kelvin. Using a spreadsheet or other computer tool to avoid the tedious calculations, calculate the maximum conversion allowed by the equilibrium at 1 atmosphere total pressure over the range of temperatures between 300 and 1000 K. Pick any suitable spacing of points for your evaluation to generate a smooth plot of f vs. T. d) Repeat the calculations for 10 and 100 atm total pressures. Show the equilibrium conversion lines for all three pressures on the same graph with appropriate labels. Comment briefly on important features of your plot.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


