Question
The amount of a radioactive isotope present in a certain sample at time t is given by A(t) = 600e-0.02826t a. Find the initial
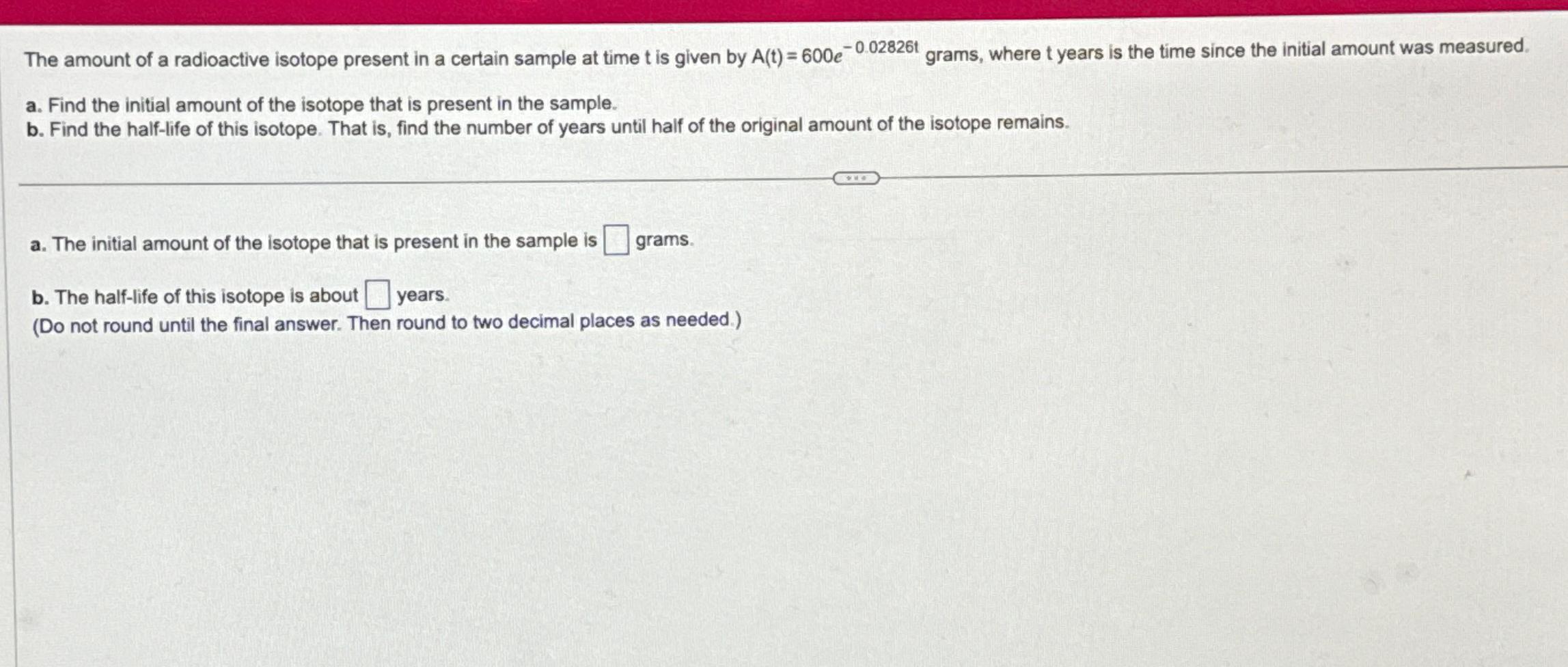
The amount of a radioactive isotope present in a certain sample at time t is given by A(t) = 600e-0.02826t a. Find the initial amount of the isotope that is present in the sample. b. Find the half-life of this isotope. That is, find the number of years until half of the original amount of the isotope remains. a. The initial amount of the isotope that is present in the sample is b. The half-life of this isotope is about years. (Do not round until the final answer. Then round to two decimal places as needed.) grams. grams, where t years is the time since the initial amount was measured.
Step by Step Solution
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
A The initial amount of the isotope that is present in the sample is given ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Precalculus
Authors: Michael Sullivan
9th edition
321716835, 321716833, 978-0321716835
Students also viewed these Mathematics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



