Question
The amount of manganese in steel is determined by changing it to permanganate ion. The steel is first dissolved in nitric acid, producing Mn2+
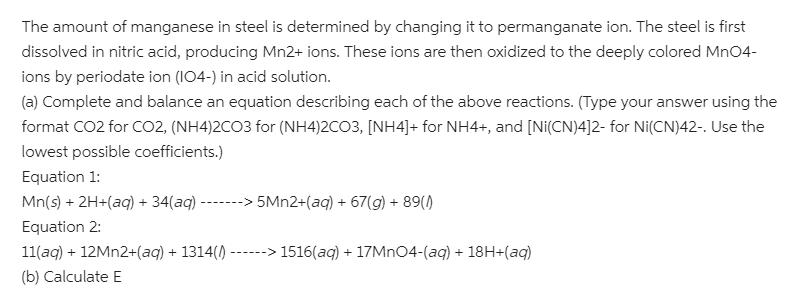
The amount of manganese in steel is determined by changing it to permanganate ion. The steel is first dissolved in nitric acid, producing Mn2+ ions. These ions are then oxidized to the deeply colored MnO4- ions by periodate ion (I04-) in acid solution. (a) Complete and balance an equation describing each of the above reactions. (Type your answer using the format CO2 for CO2, (NH4)2CO3 for (NH4)2CO3, [NH4]+ for NH4+, and [Ni(CN)4]2- for Ni(CN)42-. Use the lowest possible coefficients.) Equation 1: Mn(s) + 2H+(aq) + 34(aq) ------> 5MN2+(aq) + 67(g) + 89() Equation 2: 11(aq) + 12MN2+(aq) + 1314() --- ---> 1516(aq) + 17MN04-(aq) + 18H+(aq) (b) Calculate E
Step by Step Solution
3.51 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



