Question
THE ANSWER IS C. JUSTNEED STEP BY STEP SOLUTION. At 25C, the Ka of the hydrogen carbonate ion HCO3 is 4.8 x 10-1 and the
 THE ANSWER IS C. JUSTNEED STEP BY STEP SOLUTION.
THE ANSWER IS C. JUSTNEED STEP BY STEP SOLUTION.
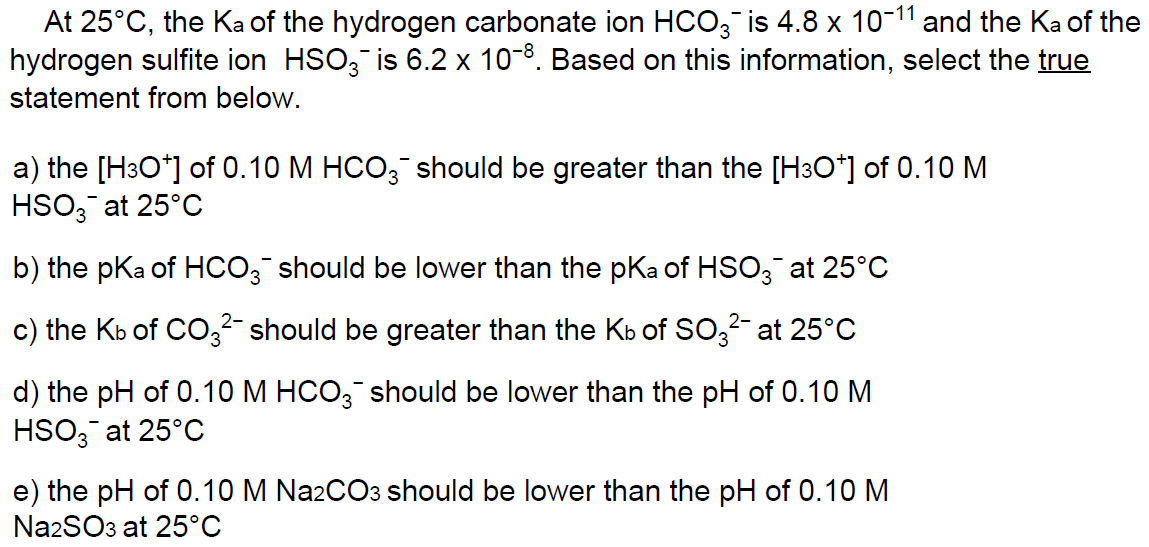
At 25C, the Ka of the hydrogen carbonate ion HCO3 is 4.8 x 10-1 and the Ka of the hydrogen sulfite ion HSO3 is 6.2 x 10-8. Based on this information, select the true statement from below. a) the [H3O+] of 0.10 M HCO3 should be greater than the [H3O+] of 0.10 M HSO3 at 25C b) the pKa of HCO3 should be lower than the pKa of HSO3 at 25C c) the Kb of CO3- should be greater than the Kb of SO3- at 25C d) the pH of 0.10 M HCO3 should be lower than the pH of 0.10 M HSO3 at 25C e) the pH of 0.10 M Na2CO3 should be lower than the pH of 0.10 M Na2SO3 at 25C
Step by Step Solution
3.41 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
Answer Given Ka H 0 40X1011 19 48X1011 11 065 1035 2 true statement Answer ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Biostatistics
Authors: Bernard Rosner
8th Edition
130526892X, 978-1305465510, 1305465512, 978-1305268920
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



