Answered step by step
Verified Expert Solution
Question
1 Approved Answer
+ The Arrhenius Equation A Review | Constants | Periodic Table The Arrhenius equation shows the relationship between the rate constant k and the temperature
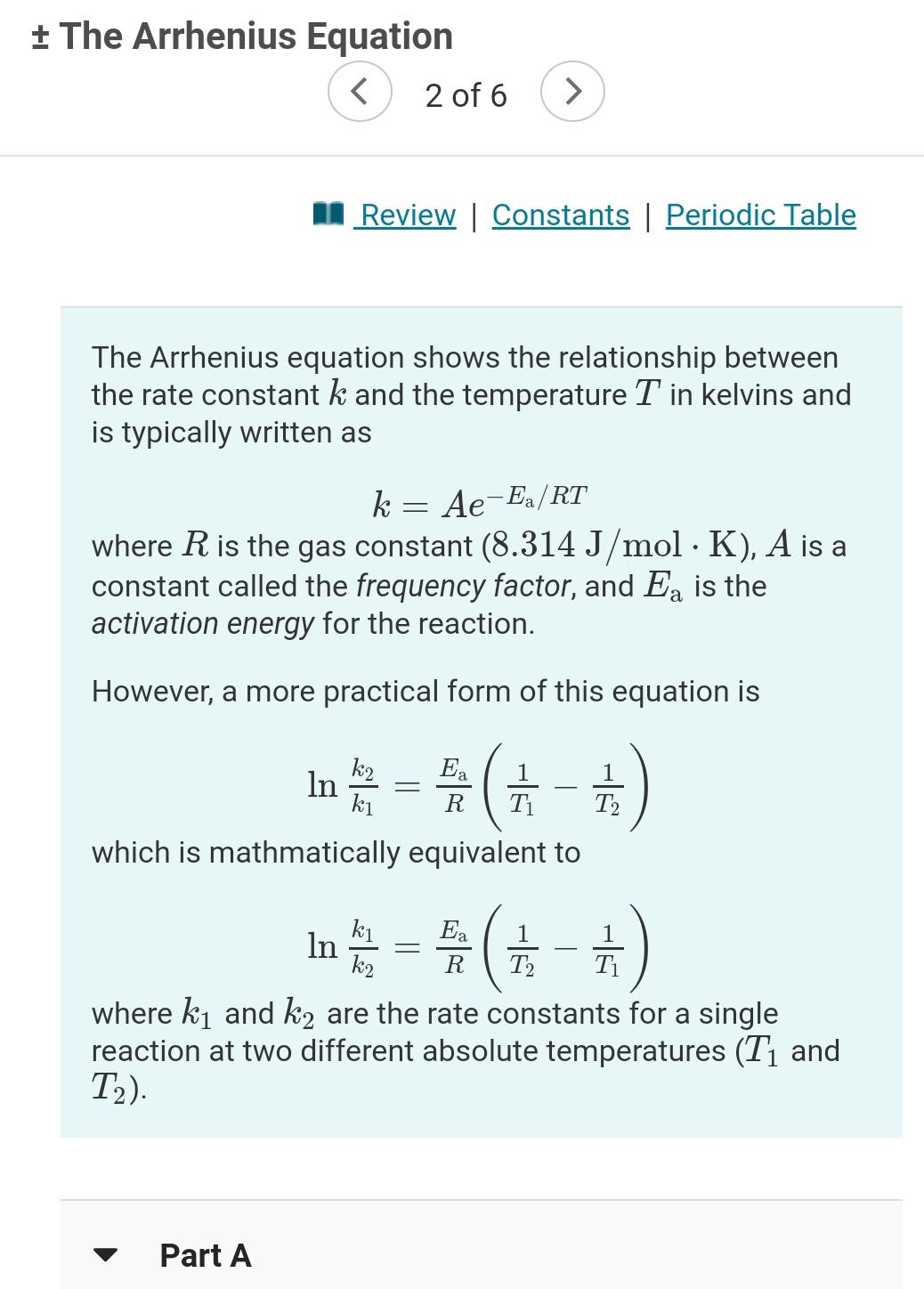
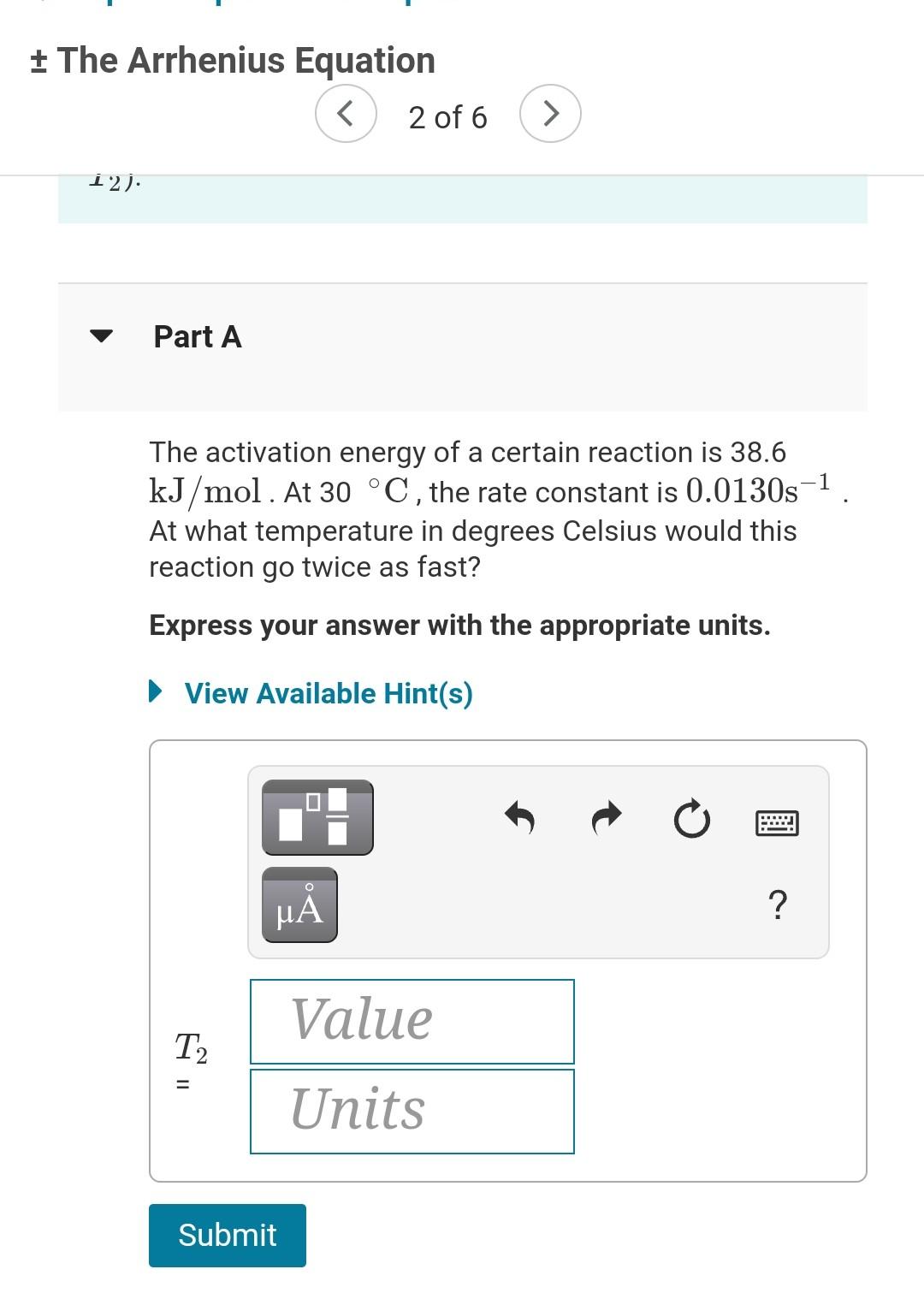
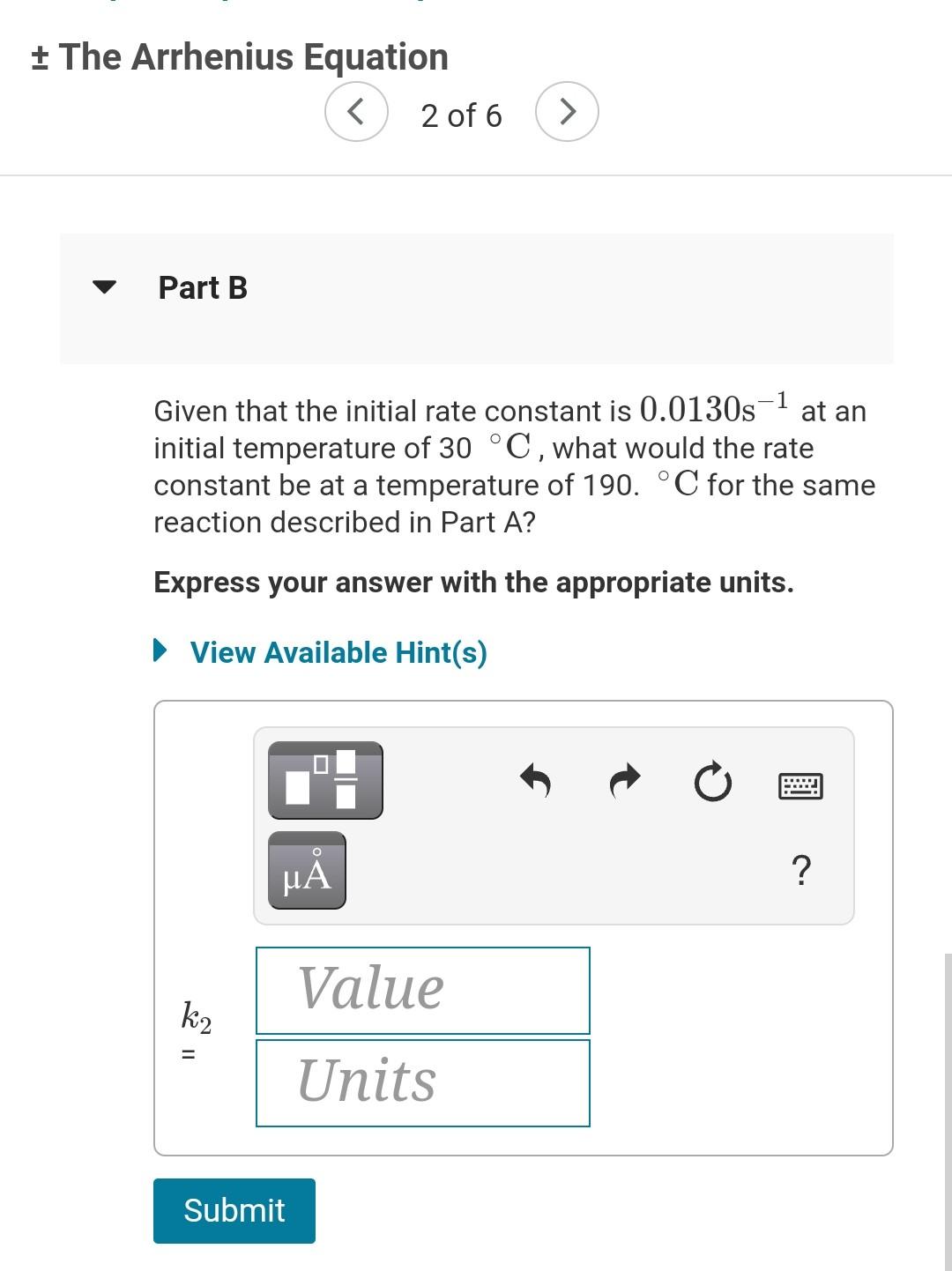
+ The Arrhenius Equation A Review | Constants | Periodic Table The Arrhenius equation shows the relationship between the rate constant k and the temperature T in kelvins and is typically written as = k = Ae-Ea/RT where R is the gas constant (8.314 J/mol K), A is a constant called the frequency factor, and Ea is the activation energy for the reaction. However, a more practical form of this equation is = Ea R In ( 1 - 1 Ti which is mathmatically equivalent to ki T2 1 1 in e = *(+-+) ( R ki Ea In k2 T2 T where k and k2 are the rate constants for a single reaction at two different absolute temperatures (T1 and T2). Part A + The Arrhenius Equation 12) Part A 1 The activation energy of a certain reaction is 38.6 kJ/mol. At 30 C, the rate constant is 0.0130s-1 At what temperature in degrees Celsius would this reaction go twice as fast? Express your answer with the appropriate units. View Available Hint(s) t ? Value T 11 Units Submit + The Arrhenius Equation 2 of 6 > Part B ) Given that the initial rate constant is 0.0130s-1 at an initial temperature of 30 C, what would the rate constant be at a temperature of 190. C for the same reaction described in Part A? O Express your answer with the appropriate units. View Available Hint(s) 2 ? Value k2 Units Submit
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


