Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The Arrhenius Equation is typically written as k=AeEa/RT However, the following more practical form of this equation also exists lnk1k2=REa(T11T21) where k1 and k2 are
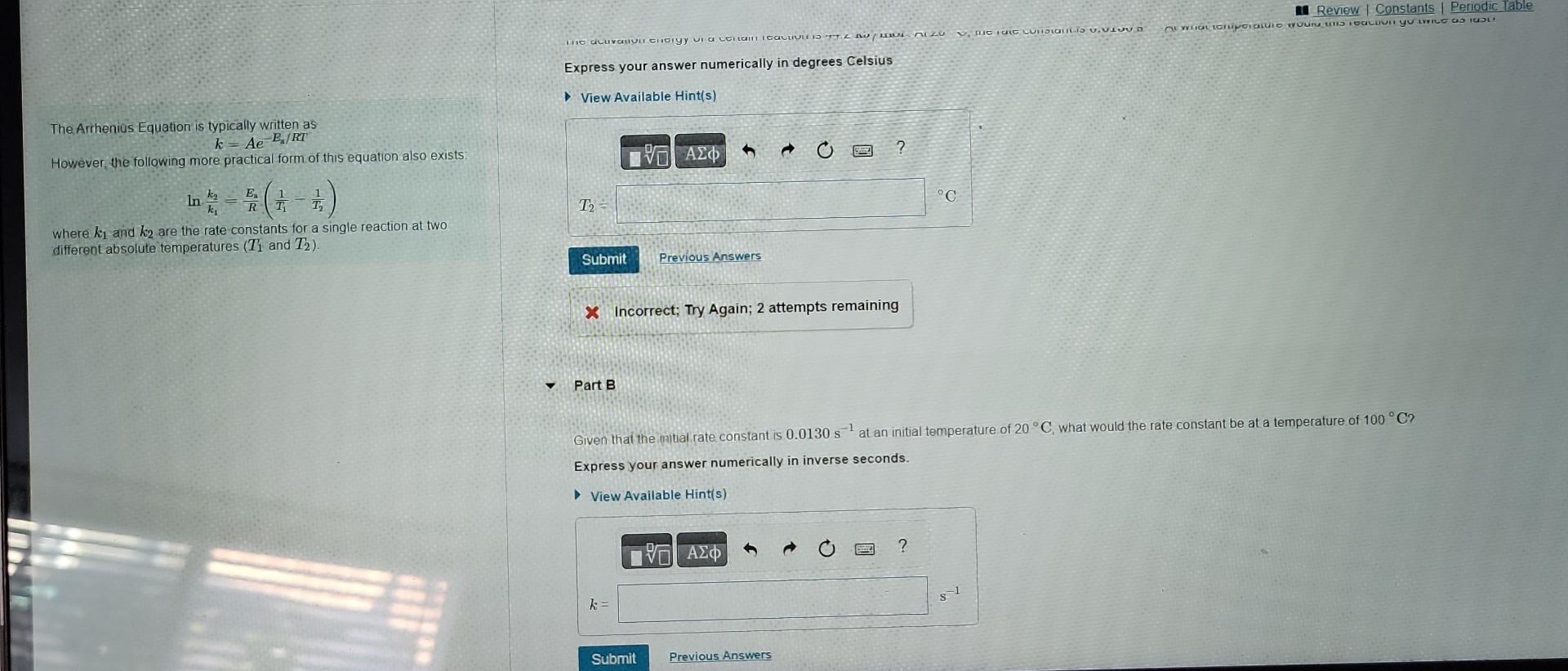
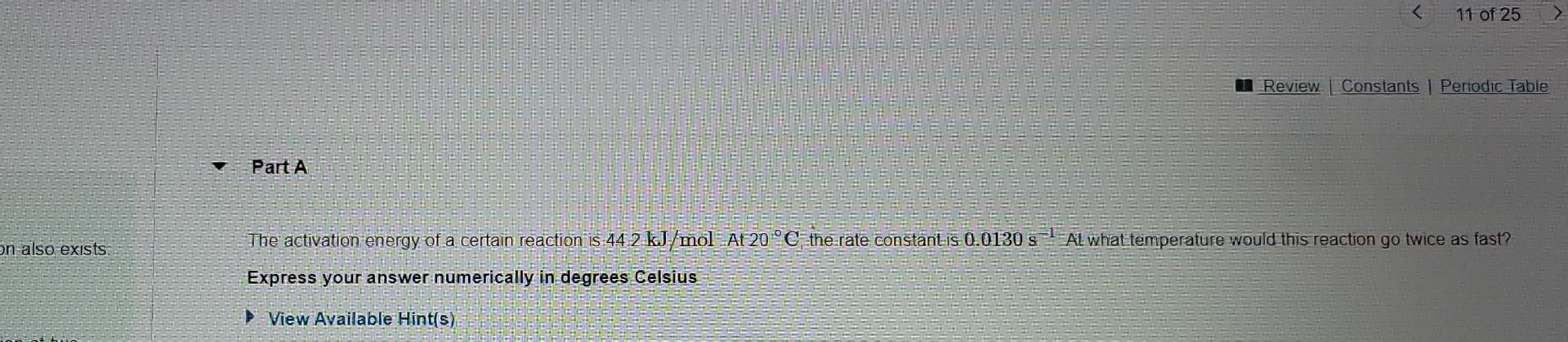
The Arrhenius Equation is typically written as k=AeEa/RT However, the following more practical form of this equation also exists lnk1k2=REa(T11T21) where k1 and k2 are the rate constants for a single reaction at two different absolute temperatures (T1 and T2 ). Express your answer numerically in degrees Celsius - Incorrect; Try Again; 2 attempts remaining Part B Given that the imitial rate constant is 0.0130s1 at an initial temperature of 20C, what would the rate constant be at a temperature of 100C ? Express your answer numerically in inverse seconds. The activation energy of a certain reaction is 44.2kJ/mol At 20C1 the rate constant is 0.0130s1 Al what temperature would this reaction go twice as fast? Express your answer numerically in degrees Celsus View Available Hint(s)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


