Answered step by step
Verified Expert Solution
Question
1 Approved Answer
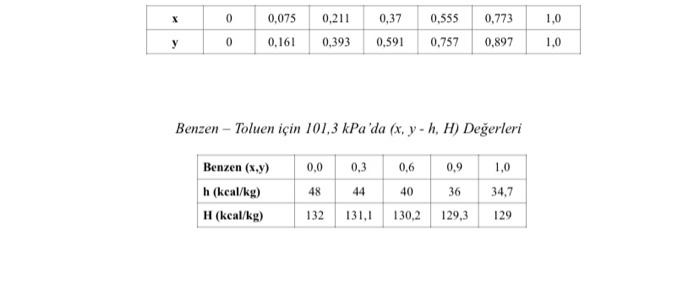
The benzene-toluene mixture containing 0.4 benzene by weight fraction is continuously preheated under pressure. This preheating is continued until the enthalpy of the liquid mixture
The benzene-toluene mixture containing 0.4 benzene by weight fraction is continuously preheated under pressure. This preheating is continued until the enthalpy of the liquid mixture becomes 72.3 kcal/kg. The mixture is then passed through a pressure reducing valve and sent to a separator at 101.3 kPa pressure conditions. By making the appropriate assumptions and using the enthalpy-composition diagram,
a) Calculate the compositions of the vapor and liquid phases obtained, b) Calculate the evaporation percentage.
Equilibrium Values at 101.3 kPa for Benzene Toluene (Weight frac.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


