Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the bottom is equation 3.3. please help (6) Consider each molecule in the table below as a one dimensional box of length L. L can
the bottom is equation 3.3. please help 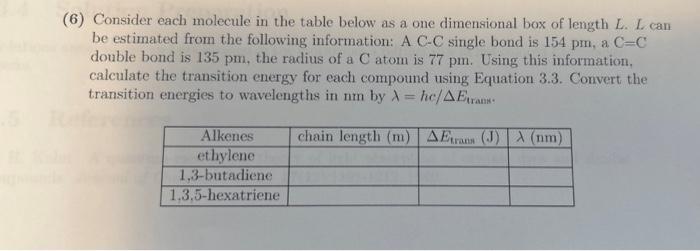
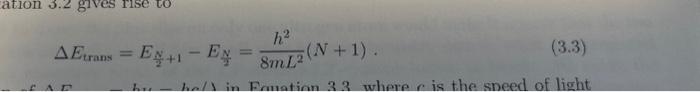
(6) Consider each molecule in the table below as a one dimensional box of length L. L can be estimated from the following information: A CC single bond is 154pm, a C=C double bond is 135pm, the radius of a C atom is 77pm. Using this information, calculate the transition energy for each compound using Equation 3.3. Convert the transition energies to wavelengths in nm by =hc/Etrans.. Etrans=E2N+1E2N=8mL2h2(N+1) 
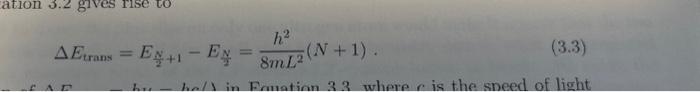
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


