
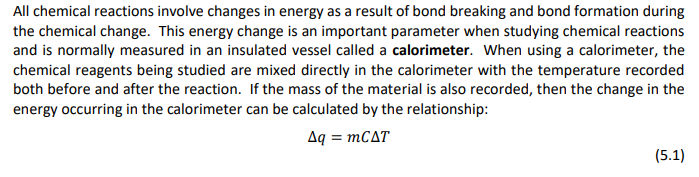
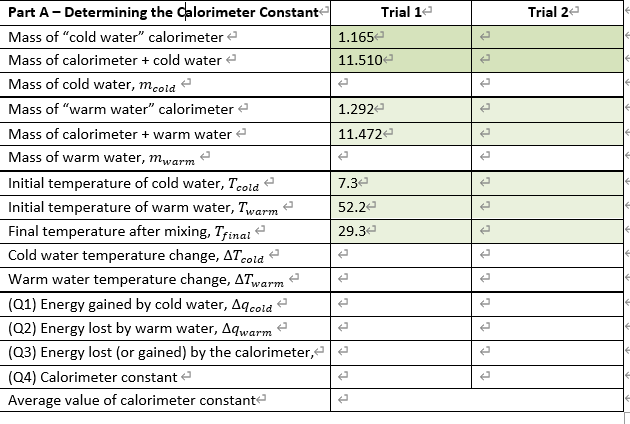

The calorimeter constant is calculated in equation 5.4 by finding the difference in the change in energy of the hot water, Aqnot , and the change in energy of the cold water, Aqcold , and dividing this difference by the temperature change which occurred during the mixing process. | Aqnot - AqcoldAqcal calorimeter constant = T| AT (5.4) All chemical reactions involve changes in energy as a result of bond breaking and bond formation during the chemical change. This energy change is an important parameter when studying chemical reactions and is normally measured in an insulated vessel called a calorimeter. When using a calorimeter, the chemical reagents being studied are mixed directly in the calorimeter with the temperature recorded both before and after the reaction. If the mass of the material is also recorded, then the change in the energy occurring in the calorimeter can be calculated by the relationship: Aq = mCAT (5.1) Trial 2 Trial 12 1.1652 11.510 1.292 11.472 Part A-Determining the calorimeter Constant Mass of "cold water" calorimeter Mass of calorimeter + cold water Mass of cold water, mcold Mass of "warm water" calorimeter Mass of calorimeter + warm water Mass of warm water, mwarm Initial temperature of cold water, Tcold Initial temperature of warm water, Twarm Final temperature after mixing, Tfinai Cold water temperature change, AT cold Warm water temperature change, ATwarm (Q1) Energy gained by cold water, A cold (Q2) Energy lost by warm water, Aqwarm (Q3) Energy lost (or gained) by the calorimeter, (24) Calorimeter constant Average value of calorimeter constante 7.32 52.2 t 29.32 tt Part A-Determining the Calorimeter Constant Calculations & Questions (Show complete sample calculation in the spaces provided and complete Table 5.1.) Q1. Using equation (5.1), calculate the energy gained by the cold water, Aqcold. Where; m = mcold, C = 4.184 J/&C, and AT = AT cold-(1.0) Q2. Using equation (5.1), calculate the energy lost by the warm water, Aqwarm. Where; m = Mwarm, C = 4.184 J/gC, and AT = ATwarm-(0.5) t Q3. Knowing that the energy lost by the calorimeter plus the energy lost by the warm water equal to the energy gained by the cold water, calculate the energy lost (or gained) by the calorimeter. (1.0) tttttt Q4. Using equation (5.4), calculate the calorimeter constant in J/C. AT = AT cold-(0.5) t Q5. The Law of Conservation of Energy states that "energy can neither be created nor destroyed but may be converted from one form to another." According to your calculations, has energy been conserved? That is, is the heat lost by the warm water equal to the heat gained by the cold water? Explain your answer in terms of your calculated results. (1.5)










