Question
The change in the mixture's Gibbs free energy as a function of composition for a Cr-Fe binary solution at 1355C is given below XFe :
The change in the mixture's Gibbs free energy as a function of composition for a Cr-Fe binary solution at 1355C is given below
XFe : 0,0 0,1 0,3 0,5 0,7 0,9 1,0
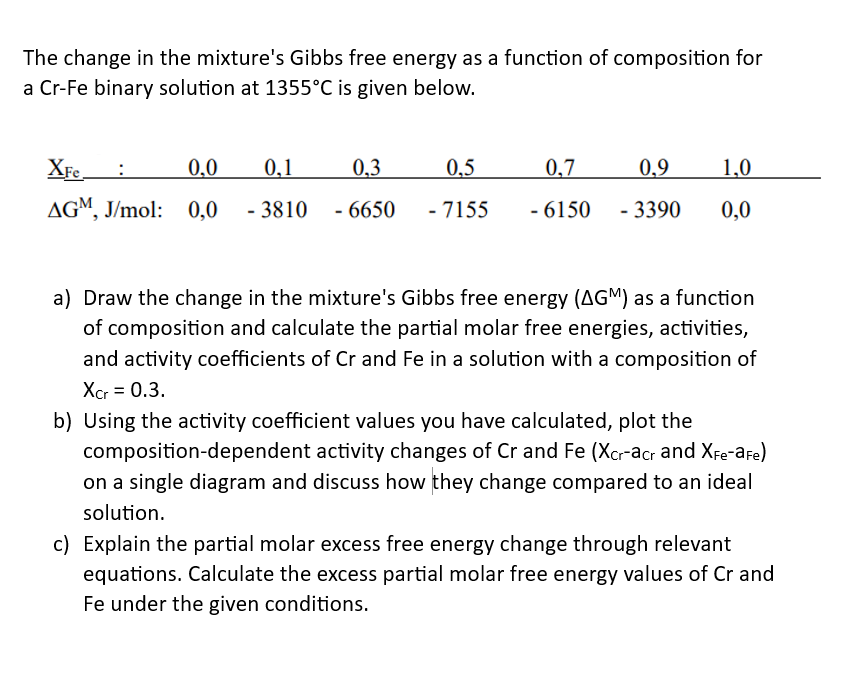 G M, J/mol: 0,0 - 3810 - 6650 - 7155 - 6150 - 3390 0,0
G M, J/mol: 0,0 - 3810 - 6650 - 7155 - 6150 - 3390 0,0
Calculate the partial molar free energies, activities, and activity coefficients of Cr and Fe in a solution
Draw the change in the mixture's Gibbs free energy (GM) as a function of composition and calculate the partial molar free energies, activities, and activity coefficients of Cr and Fe in a solution with a composition of XCr = 0.3 Using the activity coefficient values you have calculated, plot the composition-dependent activity changes of Cr and Fe (XCr-aCr and XFe-aFe) on a single diagram and discuss how they change compared to an ideal solution.
Explain the partial molar excess free energy change through relevant equations. Calculate the excess partial molar free energy values of Cr and Fe under the given conditions
The change in the mixture's Gibbs free energy as a function of composition for a Cr-Fe binary solution at 1355C is given below. a) Draw the change in the mixture's Gibbs free energy (GM) as a function of composition and calculate the partial molar free energies, activities, and activity coefficients of Cr and Fe in a solution with a composition of Xcr=0.3. b) Using the activity coefficient values you have calculated, plot the composition-dependent activity changes of Cr and Fe(XCraCCr and XFeaFe) on a single diagram and discuss how they change compared to an ideal solution. c) Explain the partial molar excess free energy change through relevant equations. Calculate the excess partial molar free energy values of Cr and Fe under the given conditions. The change in the mixture's Gibbs free energy as a function of composition for a Cr-Fe binary solution at 1355C is given below. a) Draw the change in the mixture's Gibbs free energy (GM) as a function of composition and calculate the partial molar free energies, activities, and activity coefficients of Cr and Fe in a solution with a composition of Xcr=0.3. b) Using the activity coefficient values you have calculated, plot the composition-dependent activity changes of Cr and Fe(XCraCCr and XFeaFe) on a single diagram and discuss how they change compared to an ideal solution. c) Explain the partial molar excess free energy change through relevant equations. Calculate the excess partial molar free energy values of Cr and Fe under the given conditionsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


