Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The chlorination of methane occurs in a number of steps that results in the formation of chloromethane and hydrogen chloride. The overall reaction is
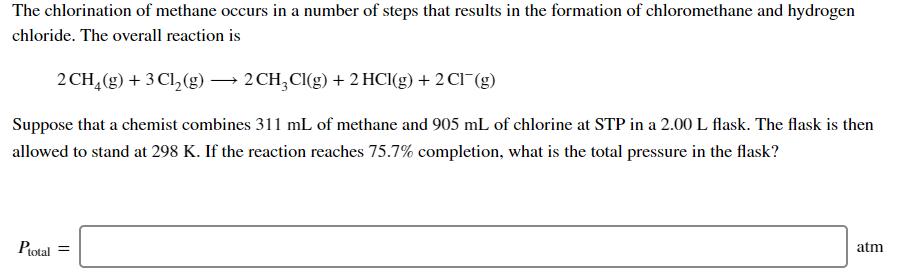


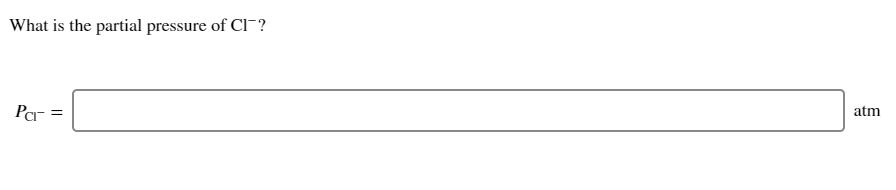
The chlorination of methane occurs in a number of steps that results in the formation of chloromethane and hydrogen chloride. The overall reaction is 2 CH4(g) + 3 Cl(g) 2 CHCl(g) + 2 HCl(g) + 2 Cl (g) Suppose that a chemist combines 311 mL of methane and 905 mL of chlorine at STP in a 2.00 L flask. The flask is then allowed to stand at 298 K. If the reaction reaches 75.7% completion, what is the total pressure in the flask? Ptotal = atm What is the partial pressure of CH4? PCH4 = What is the partial pressure of Cl? Pch2 = atm atm What is the partial pressure of CH3CI? PCHCl = What is the partial pressure of HCI? PHCI = atm atm What is the partial pressure of CI? Par = atm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


