Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The clock a) It took around 500 years to develop the perfect shape and material composition for watches. The composition used today is a
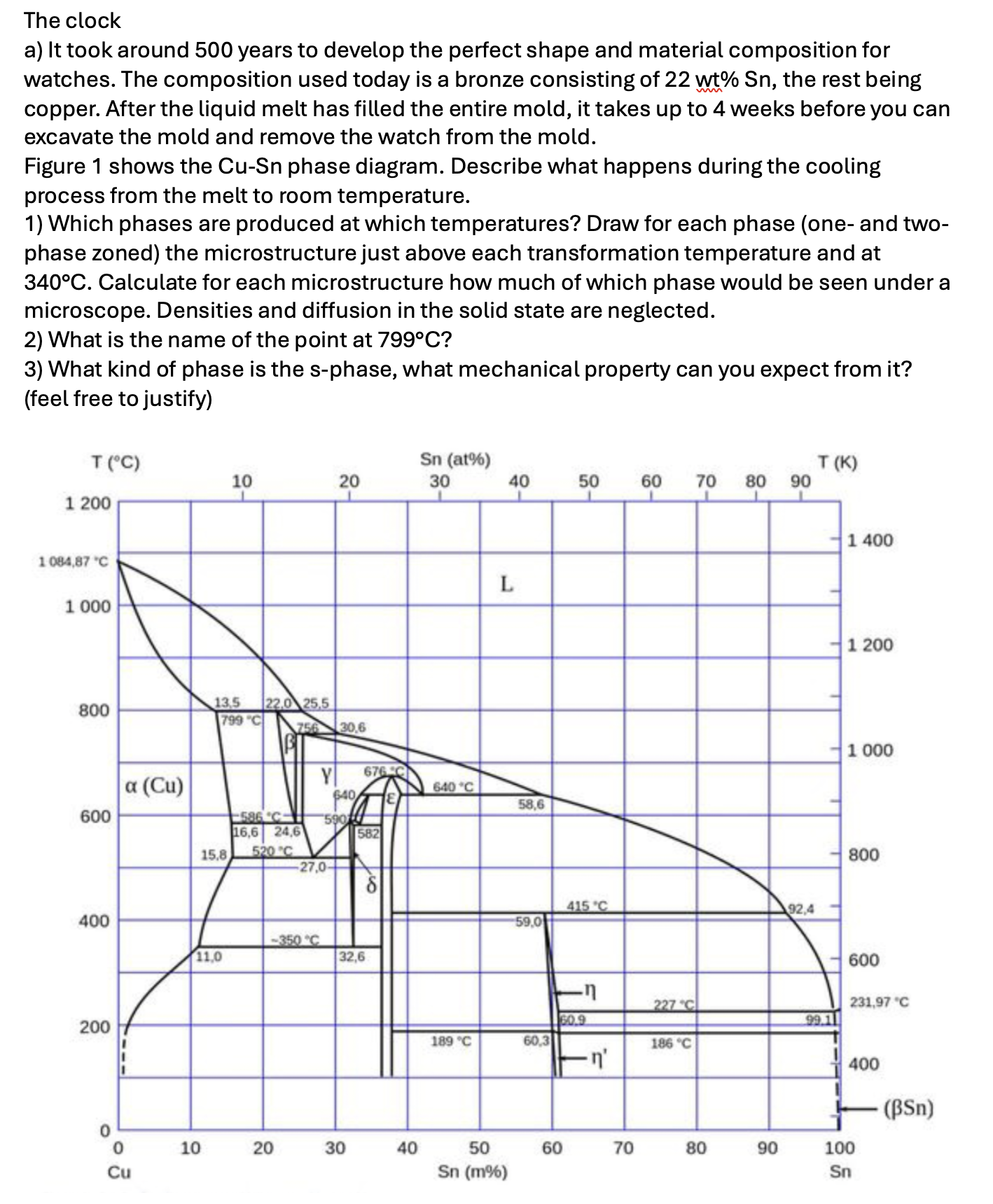
The clock a) It took around 500 years to develop the perfect shape and material composition for watches. The composition used today is a bronze consisting of 22 wt% Sn, the rest being copper. After the liquid melt has filled the entire mold, it takes up to 4 weeks before you can excavate the mold and remove the watch from the mold. Figure 1 shows the Cu-Sn phase diagram. Describe what happens during the cooling process from the melt to room temperature. 1) Which phases are produced at which temperatures? Draw for each phase (one- and two- phase zoned) the microstructure just above each transformation temperature and at 340C. Calculate for each microstructure how much of which phase would be seen under a microscope. Densities and diffusion in the solid state are neglected. 2) What is the name of the point at 799C? 3) What kind of phase is the s-phase, what mechanical property can you expect from it? (feel free to justify) T (C) 1 200 1084,87 "C 1 000 800 600 400 200 0 a (Cu) 0 Cu 15.8 13,5 22.0 25,5 799 "C 11,0 10 10 -586 C- 16,6 24,6 520 C 20 756 30,6 20 Y 676 C $40 E 27,0- 590 582 32,6 30 40 Sn (at%) 30 640 C 189 C 40 L 50 Sn (m%) 58,6 59,0 60,3 50 60 415 C -n 60.9 -n' 70 60 70 80 227 C 186 C 80 90 90 92,4 T(K) 99.1 1 400 1 200 1 000 800 600 231,97 C 400 100 Sn - (BSn)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The image provided is a CuSn coppertin phase diagram which is used to describe what happens during the cooling process of a bronze alloy containing 22 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


