Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A 200 kg chunk of lead falls from a height of 30 m and smashes into a rigid concrete floor. Calculate the increase
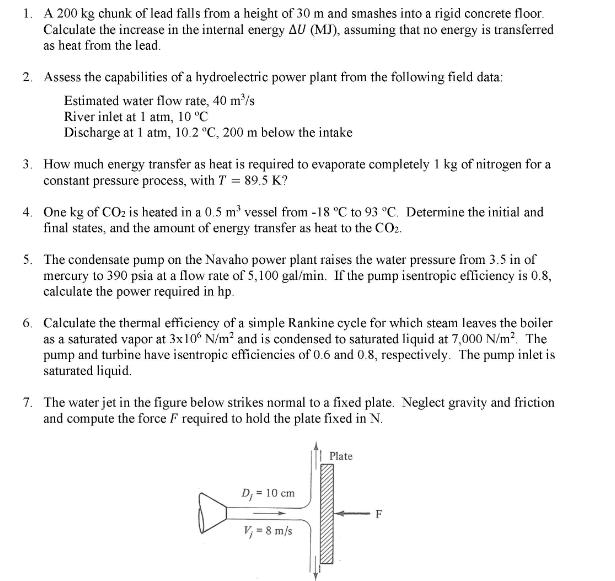
1. A 200 kg chunk of lead falls from a height of 30 m and smashes into a rigid concrete floor. Calculate the increase in the internal energy AU (MJ), assuming that no energy is transferred as heat from the lead. 2. Assess the capabilities of a hydroelectric power plant from the following field data: Estimated water flow rate, 40 m/s River inlet at 1 atm, 10 C Discharge at 1 atm, 10.2 C, 200 m below the intake 3. How much energy transfer as heat is required to evaporate completely 1 kg of nitrogen for a constant pressure process, with T = 89.5 K? 4. One kg of CO is heated in a 0.5 m vessel from -18 C to 93 C. Determine the initial and final states, and the amount of energy transfer as heat to the CO2. 5. The condensate pump on the Navaho power plant raises the water pressure from 3.5 in of mercury to 390 psia at a flow rate of 5,100 gal/min. If the pump isentropic efficiency is 0.8, calculate the power required in hp. 6. Calculate the thermal efficiency of a simple Rankine cycle for which steam leaves the boiler as a saturated vapor at 3x106 N/m and is condensed to saturated liquid at 7,000 N/m. The pump and turbine have isentropic efficiencies of 0.6 and 0.8, respectively. The pump inlet is saturated liquid. 7. The water jet in the figure below strikes normal to a fixed plate. Neglect gravity and friction and compute the force F required to hold the plate fixed in N. Plate D = 10 cm =F V = 8 m/s
Step by Step Solution
★★★★★
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Increase in Internal Energy of Lead Given Mass of lead m 200 kg Acceleration d...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


