Answered step by step
Verified Expert Solution
Question
1 Approved Answer
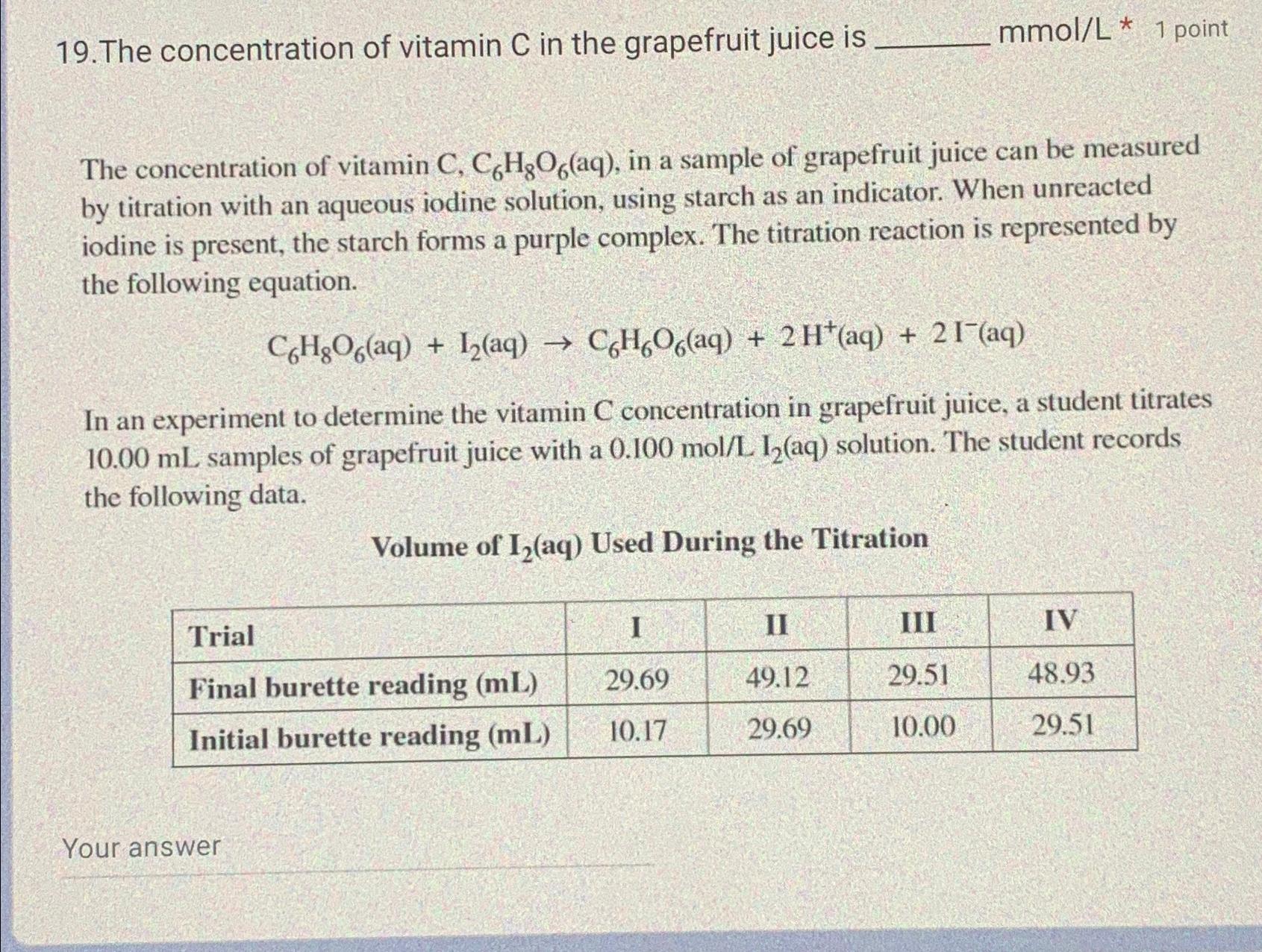
The concentration of vitamin C in the grapefruit juice is mmo l L * * 1 point The concentration of vitamin C , C 6
The concentration of vitamin in the grapefruit juice is
mmo
point
The concentration of vitamin in a sample of grapefruit juice can be measured by titration with an aqueous iodine solution, using starch as an indicator. When unreacted iodine is present, the starch forms a purple complex. The titration reaction is represented by the following equation.
In an experiment to determine the vitamin concentration in grapefruit juice, a student titrates samples of grapefruit juice with a aq solution. The student records the following data.
Volume of Used During the Titration
tableTrialI,IIIII,IVFinal burette reading Initial burette reading
Your answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


