Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The conjugate base of CH3NH2 is: * 1 point NH2- CH3NH3+ NH4+ CH3NH- Which among the compounds below 1 point is the strongest base?
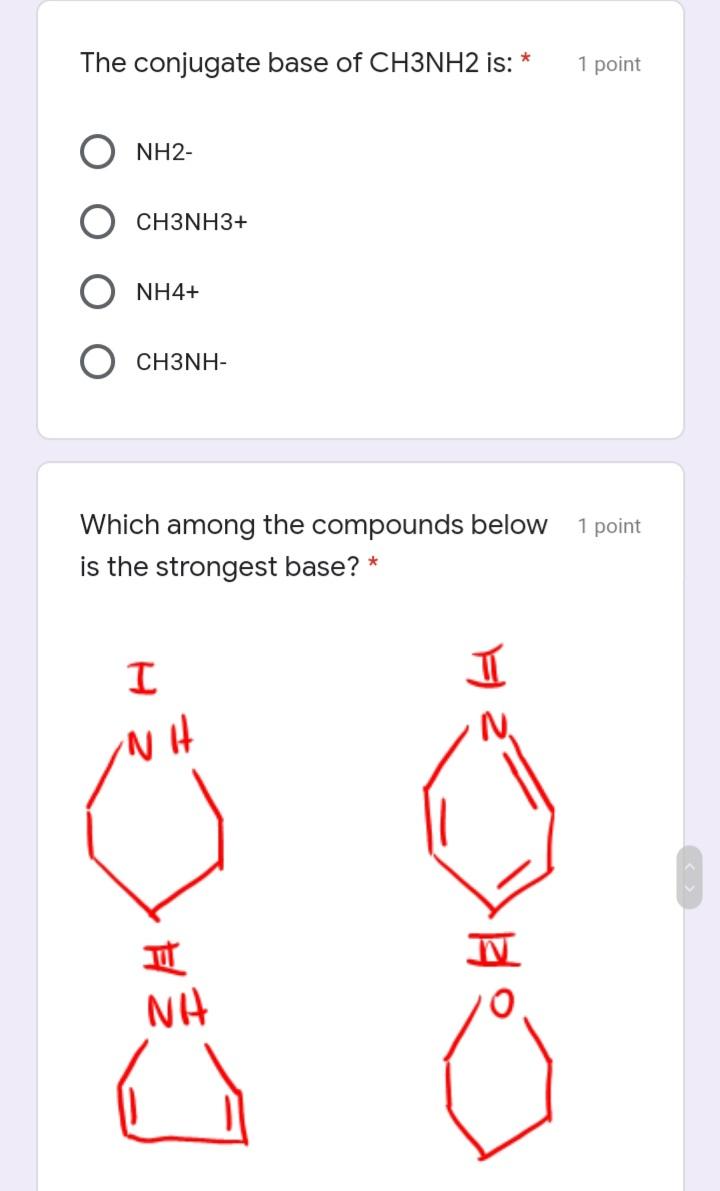

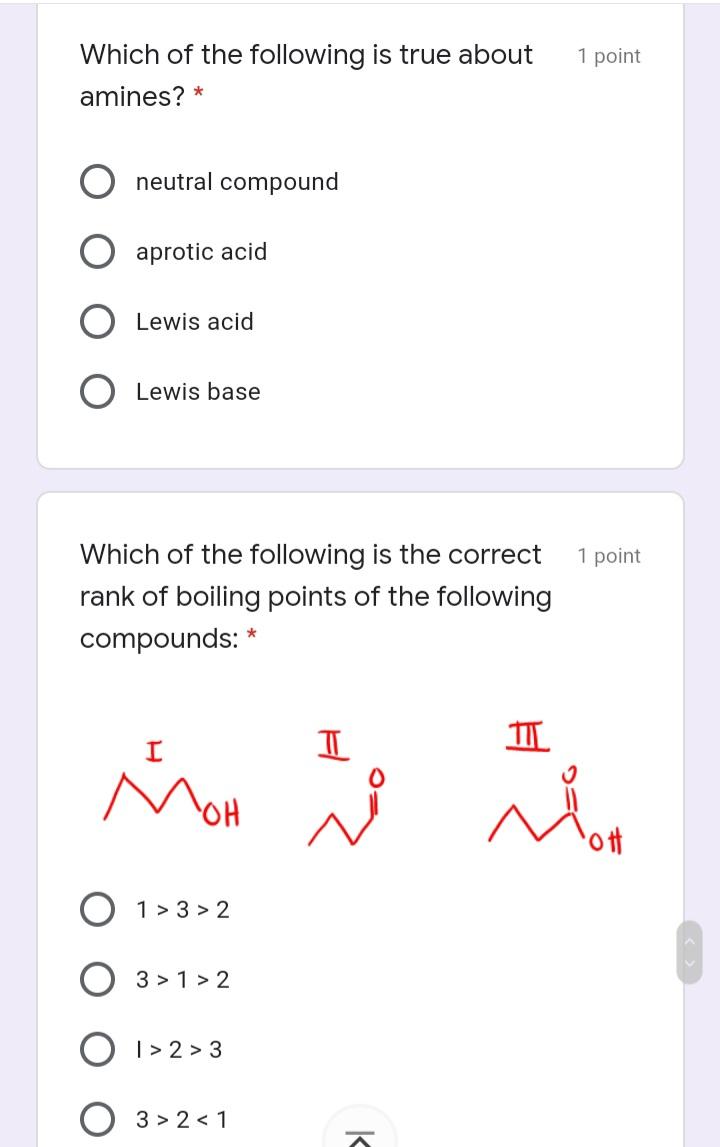
The conjugate base of CH3NH2 is: * 1 point NH2- CH3NH3+ NH4+ CH3NH- Which among the compounds below 1 point is the strongest base? * NH NH Which of the following describes the 1 point correct polarization in acrolein. (The smell of burnt fat (as when cooking oil is heated to its smoke point) is caused by glycerol in the burning fat breaking down into acrolein.) * I H2 C=CH-CH O 8* I H2 C=CH-CH=0 M H2 C-CH- CH=0 V H2 C=CHCH=0 O IV II Which of the following is true about 1 point amines? * neutral compound aprotic acid Lewis acid Lewis base Which of the following is the correct 1 point rank of boiling points of the following compounds: II MoH ott O 1> 3 > 2 3 > 1 > 2 O I> 2 > 3 3 > 2 < 1
Step by Step Solution
★★★★★
3.50 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
1 Option D Conjugate base is formed by the loss of on...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


