The hydrogen's of the hydrocarbons can b randomly replaced by chlorine by reaction of the hydrocarbon with
Question:
The hydrogen's of the hydrocarbons can b randomly replaced by chlorine by reaction of the hydrocarbon with Cl2 in the presence of light. An unknown compound, A, with the formula C4H10, produces two isomeric monochlroides, B and C, with the formula C4H9Cl, when submitted to these reaction conditions. Monochlroides B produces three isomeric dichlorides, D, E, and F, with the formula C4H8Cl2, when submitted to these reactions conditions. Monochlroides C gives a single dichloride, D, when submitted to these reaction conditions. Show the structures of A, B, C, D, E, and F. Are there any ambiguities in these structure assignments?
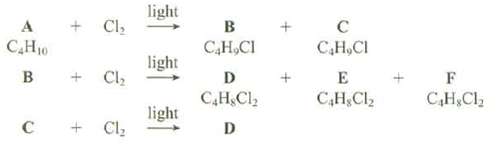
light + Cl, B С Н.СI САНю С.Н, СI light B Cl, E D С.Н.С, С,Н, С, С,Н, С. light Cl, D +
Step by Step Answer:
Based on these experiments it cannot be determined wh...View the full answer



Related Video
Using a few basic physics principles, you can impress your friends with a trick that makes a bottle disappear. For this experiment, you will need a mini plastic bottle, glycerin, a glass, and a funnel. First, open the bottle and pour some glycerin into it, then close the bottle tightly. Next, pour some water into the glass using a funnel. Place the mini bottle into the glass of water and it will look normal. This is because light travels through air faster than it travels through the glass and water, allowing our eyes to see the bottle inside the glass. However, when you fill the mini bottle with more glycerin, and pour glycerin into the glass, then put the glycerin filled bottle into the glass with glycerin in it. Half of the bottle that is submerged in the glycerin will become invisible as the light travels through glass and glycerin at the same speed, thus it does not bend and no refraction takes place, making the bottle invisible. This happens because both glass and glycerin have almost the same refractive index, which causes the speed of light to be the same in both mediums, causing no bending of light and making the bottle disappear.
Students also viewed these Organic Chemistry questions
-
(a) An unknown compound A has the formula C 7 H 12 O 2 and infrared spectrum A (p. 917). To which class does this compound belong? (b) Use the other spectra (NMR-B, p. 917, and F, p. 919; IR-D, E,...
-
An unknown compound A of molecular formula of C10H180 reacts with H2S04 and heat to form two compounds (B and C) of molecular formula C10H16. B and C both react with H2 over Pd/C to form decalin....
-
An unknown compound gives a mass spectrum with a weak molecular ion at m/z 113 and a prominent ion at m z 68. Its NMR and IR spectra are shown here. Determine the structure, and show how it is...
-
A mixture of 1 kmol carbon dioxide, 2 kmol carbon monoxide, and 2 kmol oxygen, at 25C, 150 kPa, is heated in a constant pressure steady state process to 3000 K. Assuming that only these same...
-
Arlington Corporation's financial statements (dollars and shares are in millions) are provided here Income Statement for Year Ending December 31, 2016...
-
In Problems 1-3, use the definition of a logarithmic function to rewrite each equation in exponential form. 1. 4 = log2 16 2. 4 = log3 81 3. 1 / 2 = log4 2
-
Refer to Fortune (Oct. 25,1999) magazine's study of the 50 most powerful women in America. Exercise 2.36 (p. 59). The data table, reproduced below, gives the aye (in years) and title of each of these...
-
Two stars of masses M and m, separated by a distance d, revolve in circular orbits about their center of mass (Fig. P13.69). Show that each star has a period given by T2 = 4π2d3/G (M + m) Proceed...
-
Actual revenues for the month were reported at $10,000, $2,300 under budget. This variance can definitely be attributed to...... Question 47 options: decreased unit selling prices decreases effort by...
-
Second National Insurance Company provided this information for its minority-passive equity securities: Required: 1. Provide the journal entries to record the fair value adjustment on December 31,...
-
To find a base that is strong enough to deprotonate benzoic acid but not p-methyl phenol. Then explain how this base might be used to separate these two compounds in the laboratory.
-
Provide structures for these naturally occurring compounds: (a) 2, 6-Diaminohexanoic acid (lysine, an amino acid) (b) Hex-2-en-1-yl acetate (sex attractant of Indian water bug) (c) (Z)-7-Dogecen-1-yl...
-
In order to compare the means of two populations, inde-CE1 pendent random samples of 400 observations are selected from each population, with the following results: a. Use a 95% confidence interval...
-
Suppose youre applying a simulated annealing algorithm to a certain problem, where T is the parameter that measures the tendency to accept the current candidate to be the next trial solution. You...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Assume, further, that the acquisition was consummated on October 1, 2024, as described above. However, by the end of 2025, Ayayai was concerned that the fair values of one or both of the acquired...
-
You have been asked to prepare a brief presentation on a criminological topic or issue of interest to you. Go to the Bureau of Justice Statistics (BJS) Publications & Products Overview page (See link...
-
Sparta Fashions owns four clothing stores, where it sells a wide range of women's fashions, from casual attire to formal wear. In addition, it rents formal wear and gowns for special occasions. At...
-
provide a basic analysis of financial statements;
-
Two mutually exclusive investment alternatives are being considered. Alternative A requires an initial investment of $20,000 in a machine. Annual operating and maintenance costs are anticipated to be...
-
Suppose the government passes an immigration reform law that is successful in preventing most illegal immigrants from getting employment in the United States. Which American groups would benefit and...
-
The DNA of sea urchins contains about 32% A. What percentages of the other three bases would you expect in sea urchin DNA? Explain.
-
The codon UAA stops protein synthesis. Why does the sequence UAA in the following stretch of mRNA not cause any problems? -GCA-UUC-GAG-GUA-ACG-CCC-
-
Which of the following base sequences would most likely he recognized by a restriction (endonuclease? Explain. (a) GAATTC (b) GATTACA (c) CTCGAG
-
DETAILS 1. [-/1 Points) SMITHNM13 11.2.025. MY NOTES Convert the credit card rate to the APR. Oregon, 2% per month % Need Help? ReadIt Watch
-
Corom Stack Standard CALCULATOR PRINTER VERSION BACK NEXT Problem 13-02A a-c (Part Level Submission) Sheffield Corporation had the following stockholders' equity accounts on January 1, 2020: Common...
-
Suppose that you own 2,100 shares of Nocash Corp. and the company is about to pay a 25% stock dividend. The stock currently sells at $115 per share. a. What will be the number of shares that you hold...

Study smarter with the SolutionInn App


