Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The copper is present in the slag as dissolved Cu2O and in the alloy as dissolved elemental copper. The reaction representing the equilibrium process
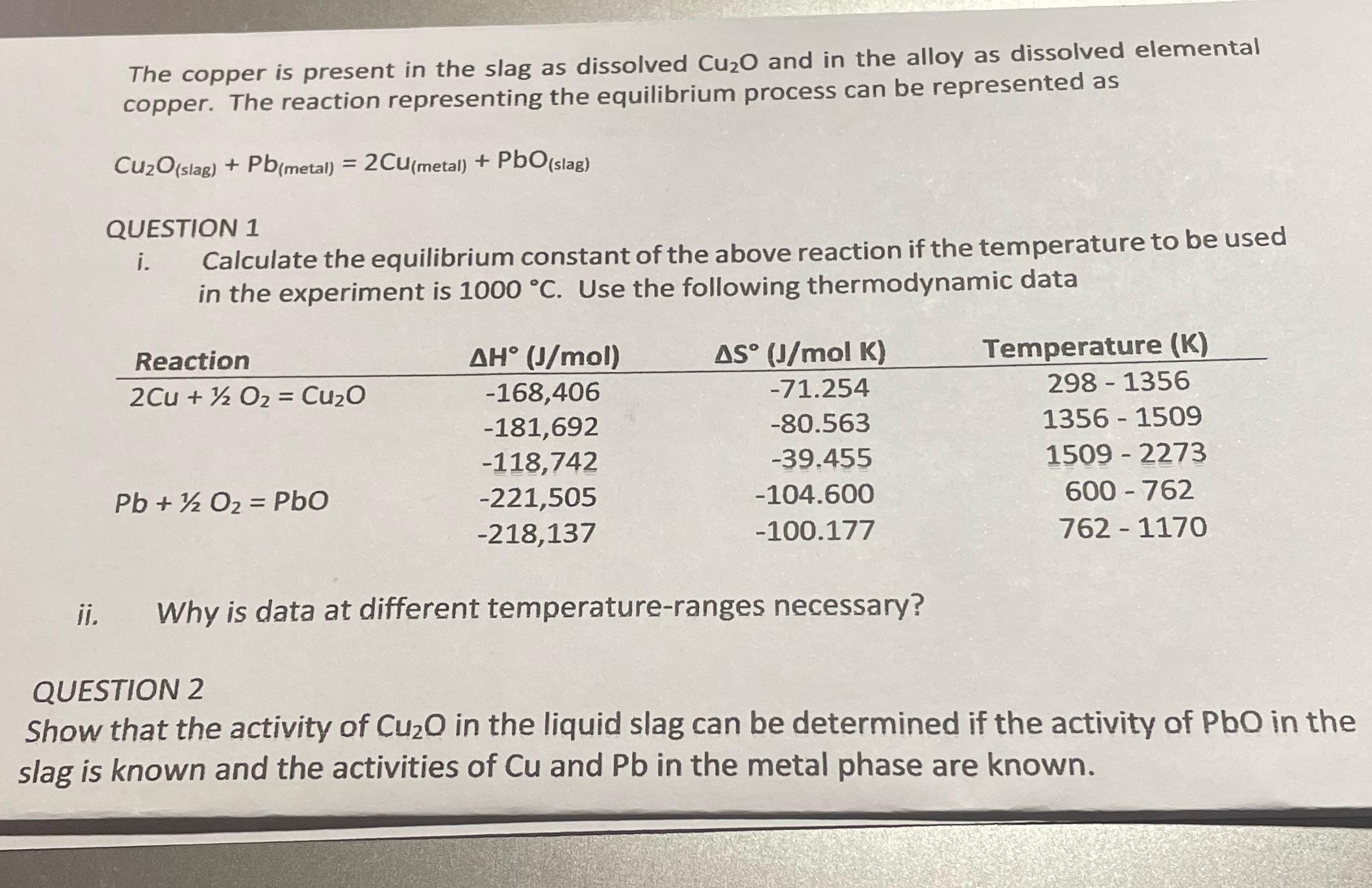
The copper is present in the slag as dissolved Cu2O and in the alloy as dissolved elemental copper. The reaction representing the equilibrium process can be represented as Cu2O(slag) + Pb(metal) = 2CU(metal) + PbO (slag) QUESTION 1 i. Calculate the equilibrium constant of the above reaction if the temperature to be used in the experiment is 1000 C. Use the following thermodynamic data Reaction 2Cu + O2 = CuO Pb+ O2 = PbO AH (J/mol) -168,406 -181,692 -118,742 -221,505 -218,137 Temperature (K) 298-1356 1356 1509 AS (J/mol K) -71.254 -80.563 -39.455 -104.600 -100.177 1509 2273 600-762 762-1170 ii. Why is data at different temperature-ranges necessary? QUESTION 2 Show that the activity of Cu2O in the liquid slag can be determined if the activity of PbO in the slag is known and the activities of Cu and Pb in the metal phase are known.
Step by Step Solution
★★★★★
3.34 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
To calculate the equilibrium constant of the given reaction at 1000C we need to use the thermodynamic data provided for the relevant reactions and app...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


