Question
The correct option(s) about entropy (S) is(are) [R = gas constant, F = Faraday constant, T = Temperature] dEcell dT an entropy driven process.
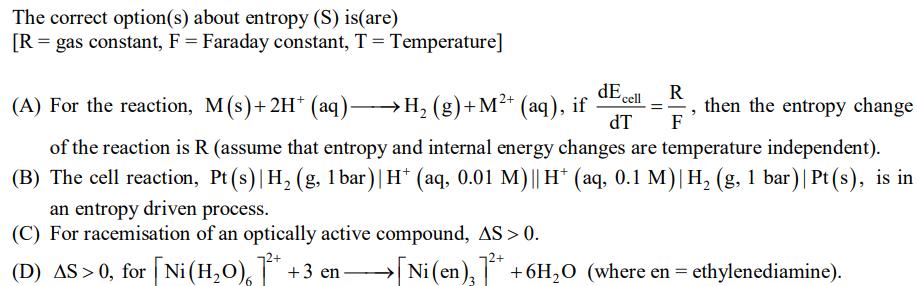
The correct option(s) about entropy (S) is(are) [R = gas constant, F = Faraday constant, T = Temperature] dEcell dT an entropy driven process. (C) For racemisation of an optically active compound, AS > 0. (D) AS > 0, for [Ni(HO) * +3 en R F (A) For the reaction, M(s) + 2H* (aq) H (g)+M+ (aq), if then the entropy change of the reaction is R (assume that entropy and internal energy changes are temperature independent). (B) The cell reaction, Pt(s)| H (g, 1 bar) | H+ (aq, 0.01 M) || H+ (aq, 0.1 M)| H (g, 1 bar) | Pt(s), is in [Ni(en) + + 6HO (where en = ethylenediamine).
Step by Step Solution
3.32 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



