Answered step by step
Verified Expert Solution
Question
1 Approved Answer
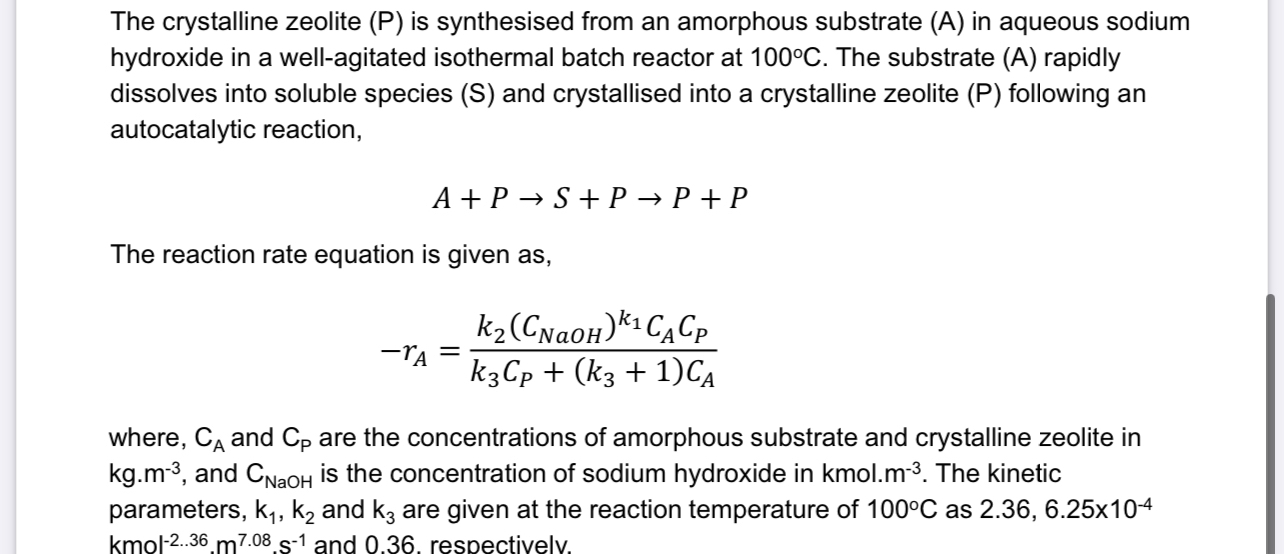
The crystalline zeolite ( P ) is synthesised from an amorphous substrate ( A ) in aqueous sodium hydroxide in a well - agitated isothermal
The crystalline zeolite is synthesised from an amorphous substrate in aqueous sodium hydroxide in a wellagitated isothermal batch reactor at The substrate A rapidly dissolves into soluble species and crystallised into a crystalline zeolite following an autocatalytic reaction,
The reaction rate equation is given as
where, and are the concentrations of amorphous substrate and crystalline zeolite in and is the concentration of sodium hydroxide in The kinetic parameters, and are given at the reaction temperature of as and respectivelv,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


